b ESCOM, UTC, EA TIMR 4297, 1 allée du Réseau Jean-Marie Buckmaster, 60200 Compiègne, France;
c Sorbonne Universités, Université de Technologie de Compiègne, Génie Enzymatique et Cellulaire(GEC), UMR-CNRS 7025, CS 60319, 60203 Compiègne Cedex, France;
d Laboratoire de chimie durable et santé, Yncrea Hauts-de-France, HEI, 13 rue de Toul, 59046 Lille Cedex, France
Botrytis cinerea is mainly responsible for the so-called grey rot on various plants. Principal targets are onions [1], beans [2], tomatoes [3], but also grapevines [4, 5] and over 230 plant species over the world [6]. Unfortunately, resistance phenomena were observed towards various treatments. Botrytis cinerea tolerates chlorinated nitrobenzene antifungals [7] as well as benzimidazoles [8], and dicarboxymides [5, 9]. However, molecules containing phenol and/or alkoxybenzenes are commercialized for their antifungal activity against Botrytis cinerea (Fig. 1).
|
Download:
|
| Fig. 1. Active ingredients of A: Mevalone (SUMIAGRO); B: Kenja (SUMMIT AGRO); C: TeldorⓇ (BAYER) [10]. | |
New chemicals based on these phenols and/or alkoxybenzenes cores can be synthesized, using as much as possible eco-friendly conditions and bioresources to obtain these chemicals. In this context, salicylaldehyde was first discovered in 1838, from Filipendula vulgaris [11] and possessed simultaneously a phenol group that might be interesting against Botrytis cinerea and an aldehyde moiety, which is a useful group for functionalization especially into ether group. For this ether formation from carbonyl moiety can be cited for example, the use of functionalized silanes and catalysts [12-14], of frustrated Lewis pair catalysts hydrogenation with alkyl orthoformate [15], or of tin reagents [16]. The reductants can be also either complex hydrides [17] or dihydrogen on Pd/Ru [18, 19]. But to the best of our knowledge, no zinc reaction in polar solvents was used to achieve this reaction. Moreover, reduction of carbonyl compounds thanks to zinc-mercury amalgam is well-known as Clemmensen reaction [20]. But Clemmensen-like reactions in the absence of mercury remain discreet [21, 22]. Other reactions leading to hydrocarbons from carbonyls can be used [23], as for example Huang-Minion modification of Wolff-Kishner reduction under microwave irradiation [24, 25]. However zinc, by its low cost, its good performances in reduction or coupling and its compatibility with many solvents is a prime reagent to obtain biocides from o-salicylaldehyde.
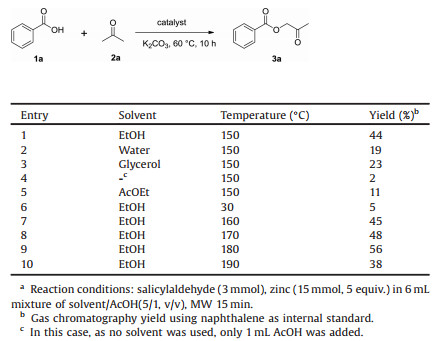
First, zinc-mediated Clemmensen reduction was performed on o-salicylaldehyde (1) by using green solvents as ethanol, water, glycerol or without solvent. These reactions were performed under microwave irradiation, and reaction temperature was modulated from 150 ℃ to 190 ℃.
From Table 1 can be deduced that amongst the green solvent used (EtOH, water, glycerol, entries 1-3) or without solvent (entry 4), ethanol is the best solvent for the reaction leading to 44% isolated yield at 150 ℃. Indeed, when water was used as solvent (entry 2) or if no solvent was added (entry 4), miscibility problem between acetic acid and salicylaldehyde was observed. To investigate whether the content of AcOEt at the end of reaction was responsible for the good yield with EtOH, a reaction with AcOEt as solvent was performed (entry 5) and led only to a yield of 11% in compound 2 leading to the conclusion that AcOEt was not responsible for the yields obtained with EtOH. Then the reaction performed at 30 ℃ (entry 6) only led to 5% yield, whereas at 160 ℃ (entry 7) and 170 ℃ (entry 8) the yields obtained were just below 50%. Increasing the temperature to 180 ℃ (entry 9) seemed to be the best one leading to 56% yield, whereas at 190 ℃ (entry 10), the molecule 2 was only obtained in 38% yield.
|
|
Table 1 Optimization of the reaction conditions.a |
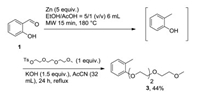
Then, under microwave irradiation at 180 ℃ for 15 min, o-salicylaldehyde (1) was reduced to o-cresol and was used without purification for a nucleophilic substitution with triethylene glycol monomethyl ether [26] leading to compound 3, with an overall isolated yield of 44% (Scheme 1). This one-pot/twostep procedure is in accordance with some of the principles of green chemistry [27].
|
Download:
|
| Scheme 1. Synthesis of compound 3. | |
Indeed, if the yield of reaction remains modest, these two steps are in accordance with eco-friendly chemistry. Indeed, solvents are green-light (green, step 1) and orange-light (usable, step 2) according to Pfizer "traffic light" of environmental solvent impact [28].
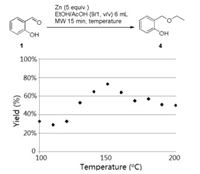
In a second time, as alkoxyphenols could have a real importance against Botrytis cinerea, zinc-mediated reductive coupling reaction between o-salicylaldehyde (1) and ethanol was performed for the reaction optimization. As seen from Fig. 2, from 100 ℃ to 150 ℃, the yields of 4 increased from 33% to 73%, and then from 150 ℃ to 200 ℃, they decreased to 50%. Indeed, because of the zinc ability to heat up to 500 ℃ under microwave irradiation, it can be supposed that above 150 ℃ in reaction media, some degradation may occur.
|
Download:
|
| Fig. 2. Synthesis of compound 4. Gas chromatography yield using naphthalene as internal standard. | |
Then, various metals such as magnesium, aluminum, iron, and copper powders were tested and compared to zinc. The results (Table 2) suggest that only zinc is reactive for the alcohol-carbonyl reductive coupling (entry 1).
|
|
Table 2 Conversion and selectivity of the reaction using various metal source.a |
Indeed, we had to take into account that some metals can perform homo-pinacol coupling (Zn, Mg), or reduction to compound 5 (Al, Fe) in addition to product 4. Mg, Al, Fe and Cu (entries 2-5) gave poor yields of product 4 (Table 2). In our case, the reaction with Mg gave homo-pinacol coupling in 24% yield, where no Pinacol coupling was observed when zinc was used. As mentioned above, one of the most known side-reaction of the envisioned one is called Bouveault-Blanc reaction, and consists in reduction of carbonyl into alcohol moiety usually using sodium as reductant. Under our best conditions (using zinc, under microwave irradiation, at 150 ℃ for 15 min.), the selectivity of compound 4 over hydroxybenzyl alcohol 5 was depending on the presence or absence of acetic acid and water. Indeed, in the presence of water but not acetic acid (entry 6), only 5% of conversion was observed, whereas in the presence of acetic acid and water (entry 7), 58% conversion was obtained, but with a lower selectivity than without water (entry 1). Moreover, as the reaction took place in the surface of the zinc powder, 5 equiv. of zinc were necessary to obtain the best yield of compound 4 (entries 1, 8-10).
For the formation of compound 4, two mechanistic pathways could be envisioned (Scheme 2): the upper one uses first ethanol in acidic conditions to obtain the oxonium ion [29]. Then zinc in acetic acid leads to the reached ether. The other pathway is the two-electron reduction of the aldehyde [30], followed by the ethanol addition. As the reaction directly performed from the benzylic alcohol 5 [31] did not succeed, that led us to suggest the upper pathway as a mechanism for the reaction (Scheme 2).

|
Download:
|
| Scheme 2. Proposed mechanism of the formation of 4. | |
To evaluate the scope of the reaction, the reaction was performed on butyl alcohol and hexyl alcohol, and hexenyl alcohol (Table 3, entries 2, 3 and 6).
|
|
Table 3 Scope of the reaction. |
It is noticeable that, due to the microwave ability to act on polar molecules, the bigger is the aliphatic chain length of alkyl alcohols, the worse is the reaction yield. So in our conditions, products 4, 6, 7 and 10 were obtained in 73%, 59%, 26% and 24%, respectively (entries 1, 2, 3 and 6). When more hindered alcohols were chosen as neopentyl alcohol (entry 4) and tert-butyl alcohol (entry 5) no attended product was obtained. Reaction on bromobenzaldehyde did not give the attended product (entry 7). Finally, as salicylaldehyde was not able in our conditions to give any homo-pinacol coupling, we tested the reaction with a ratio EtOH/AcOH (1:1, v/v) to minimize the ether formation and added hexenal (entry 8) which gave product 12 of hetero-pinacol coupling in 61% yield (entry 8). For more details about the syntheses, please see the Supporting information.
Some of the synthesized compounds were evaluated for their in vitro antifungal potential against Botrytis cinerea (MUCL000399). First the compounds were tested on the mycelium of Botrytis cinerea at a concentration of 50 μg/mL and gave the inhibition rates shown in Fig. 3. From Fig. 3 one can clearly conclude that all the compounds synthesized had a significantly better effect on Botrytis cinerea than the starting material salicylaldehyde 1. Cresol 2 had an effect just above 60%, which can be compared to ethyloxymethylphenol 4. Compound 3 seems to be less efficient than cresol 2 and butyloxymethylphenol 6 had inhibition rates of 69%. Pinacol 12 did not give any inhibitory rate, but the most active compound was clearly and significantly hexyloxymethylphenol 7 with an inhibition rate of 100%.

|
Download:
|
| Fig. 3. Inhibitory rates of Botrytis cinerea by compounds 1, 2, 3, 4, 6, 7 and 12 at a concentration of 50 μg/mL. | |
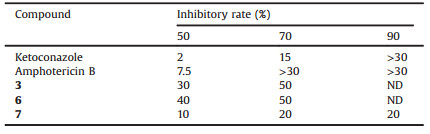
So MIC (minimum inhibitory concentration) was chosen to be performed on compounds 3, 6 and 7. As molecules having antifungal activities on plant pathogens usually act on respiration (thiram, mancozeb, captan, dichlofluanid, tolylfluanid), cytochrome b (strobilurins), β-tubulin (MBC-generating pesticides), osmoregulation (dicarboxymides), sterol biosynthesis (Fenhexamide) or hydrolytic enzymes secretion (anilinopyrimidines) [35], our molecules 3, 6 and 7 were compared to positive control standards Ketoconazole [36] and Amphotericin B [37] which were chosen due to their capability to interact with membrane ergosterol. Table 4 confirms the interest of compound 7, which enables to reach 50% and 70% of inhibition with lower concentrations than the two other tested compounds. The compound 7 even enables to reach 90% of inhibition, which was not possible with the two other molecules for the tested concentration range. Compared to positive controls, results of compound 7 remain interesting. Indeed even if Ketoconazole and Amphotericin B trigger 50% of inhibition with lower concentrations, they do not enable to reach 90% for 20 μg/mL as the compound 7 does for this fungal strain.
|
|
Table 4 MIC measurements (μg/mL) |
To conclude a series of biocides from o-salicylaldehyde by using zinc was obtained, which possesses good performances in reductive ether formation, Clemmensen-like reaction, or heteropinacol coupling. Alkoxyphenol compounds proved to be particularly interesting for Botrytis cinerea control. In this context, studies are ongoing within the team to design new antifungal agents from other bioresources.
AcknowledgmentsAuthors would like to thank ESCOM for funding, as well as Wallonie-Bruxelles International et le Fonds National de la Recherche Scientifique pour la communauté française de Belgique, Ministère des Affaires Etrangères et du Développement International (MAEDI) et Ambassade de France en Belgique pour la France and by the Region of Picardie, France (cofunding of equipment under CPER 2007–2020 project). Authors would also like to thank Ministère de l'Education Nationale de la Recherche et de la Technologie for Research fellowships.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.08.053.
| [1] |
M.I. Chilvers, L.J. du Tiot, Plant Health Prog. 7 (2006), doi: http://dx.doi.org/10.1094/PHP-2006-1127-01-DG.
|
| [2] |
Y. Li, S. Sun, C. Du, et al., Crop Prot. 85 (2016) 52-56. DOI:10.1016/j.cropro.2016.03.020 |
| [3] |
A. Wakeham, A. Langton, S. Adams, R. Kennedy, Crop Prot. 90 (2016) 27-33. DOI:10.1016/j.cropro.2016.08.014 |
| [4] |
A. Panebianco, I. Castello, G. Cirvilleri, et al., Crop Prot. 77 (2015) 65-73. DOI:10.1016/j.cropro.2015.07.010 |
| [5] |
P. Leroux, M. Clerjeau, Crop Prot. 4 (1985) 137-160. DOI:10.1016/0261-2194(85)90014-6 |
| [6] |
Y. Elad, B. Williamson, P. Tudzynski, N. Delen, Botrytis: Biology, Pathology and Control, Springer, Dordrecht, 2007, pp. 1-8.
|
| [7] |
M.J. Reavill, Ann. Appl. Biol. 41 (1954) 448-460. DOI:10.1111/j.1744-7348.1954.tb01144.x |
| [8] |
G.J. Bollen, G. Scholten, Neth. J. Plant Pathol. 77 (1971) 83-90. DOI:10.1007/BF01981496 |
| [9] |
T. Katan, Plant Pathol. 31 (1982) 133-141. DOI:10.1111/j.1365-3059.1982.tb02821.x |
| [10] |
"Fongicides TeldorⓇ: Bayer-Agri, traitement phytopharmaceutique pour la protection des cultures-TeldorⓇ, " https://www.bayer-agri.fr/produits/fiche/fongicides-teldor.
|
| [11] |
N. Radulović, M. Mišić, J. Aleksić, et al., Fitoterapia 78 (2007) 565-570. DOI:10.1016/j.fitote.2007.03.022 |
| [12] |
M.S. Yang, L.W. Xu, H.Y. Qiu, G.Q. Lai, J.X. Jiang, Tetrahedron Lett. 49 (2008) 253-256. DOI:10.1016/j.tetlet.2007.11.062 |
| [13] |
B.A. Gellert, N. Kahlcke, M. Feurer, S. Roth, Chem.-Eur. J. 17 (2011) 12203-12209. DOI:10.1002/chem.201101819 |
| [14] |
G.L. Larson, J.L. Fry, Org. React. 71 (2008) 1-737. |
| [15] |
M. Bakos, Á. Gyömöre, A. Domján, T. Soós, Angew. Chem. Int. Ed. 56 (2017) 5217-5221. DOI:10.1002/anie.201700231 |
| [16] |
J.P. Quintard, B. Elissondo, D. Mouko-Mpegna, J. Organomet. Chem 251 (1983) 175-187. DOI:10.1016/S0022-328X(00)99500-7 |
| [17] |
P.H.J. Carlsen, M. Ystenes, U. Ragnarsson, T. Anthonsen, R. Kivekäs, Acta Chem. Scand. 40b (1986) 757-759.
|
| [18] |
R.M. Mironenko, O.B. Belskaya, T.I. Gulyaeva, et al., Catal. Today 279 (2017) 2-9. DOI:10.1016/j.cattod.2016.07.022 |
| [19] |
R.M. Mironenko, O.B. Belskaya, V.I. Zaikovskii, V.A. Likholobov, Monatshefte Für Chem.-Chem. Mon. 146 (2015) 923-930. DOI:10.1007/s00706-015-1445-4 |
| [20] |
E. Clemmensen, Berichte Dtsch. Chem. Ges. 46 (1913) 1837-1843. DOI:10.1002/cber.19130460292 |
| [21] |
S. Yamamura, S. Ueda, Y. Hirata, Chem. Commun. (1967) 1049-1050.
|
| [22] |
S. Yamamura, Y. Hirata, J. Chem. Soc. C (1968) 2887-2889.
|
| [23] |
B.P. Bandgar, S.N. Kshirsagar, P.P. Wadgaonkar, Synth. Commun. 25 (1995) 941-945. DOI:10.1080/00397919508012655 |
| [24] |
P. Jaisankar, B. Pal, V.S. Giri, Synth. Commun. 32 (2002) 2569-2573. DOI:10.1081/SCC-120005941 |
| [25] |
S. Chattopadhyay, S.K. Banerjee, A.K. Mitra, J. Indian Chem. Soc. 79 (2002) 906-907. |
| [26] |
M. Yoshimoto, K. Honda, S. Kurosawa, M. Tanaka, J. Phys. Chem. C 118 (2014) 16067-16073. DOI:10.1021/jp411186n |
| [27] |
P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, 1998.
|
| [28] |
C. Reichardt, T. Welton, Solvents and Solvent Effects in Organic Chemistry, 4th Ed., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2010.
|
| [29] |
C. Zhao, C.A. Sojdak, W. Myint, D. Seidel, J. Am. Chem. Soc. 139 (2017) 10224-10227. DOI:10.1021/jacs.7b05832 |
| [30] |
T. Hirao, S. Santhitikul, H. Takeuchi, A. Ogawa, H. Sakurai, Tetrahedron 59 (2003) 10147-10152. DOI:10.1016/j.tet.2003.10.068 |
| [31] |
A.R. Surrey, Name Reactions in Organic Chemistry, 2nd Ed., Academic Press, 1961, pp. 27-28.
|
| [32] |
Y. Herzig, L. Lerman, W. Goldenberg, et al., J. Org. Chem. 71 (2006) 4130-4140. DOI:10.1021/jo052621m |
| [33] |
A.R. Katritzky, Z. Zhang, X. Lan, H. Lang, J. Org. Chem. 59 (1994) 1900-1903. DOI:10.1021/jo00086a050 |
| [34] |
M.T. Richers, M. Breugst, A.Y. Platonova, et al., J. Am. Chem. Soc. 136 (2014) 6123-6135. DOI:10.1021/ja501988b |
| [35] |
B. Williamson, B. Tudzynski, P. Tudzynski, J.a.L.V. Kan, Mol. Plant Pathol. 8 (2007) 561-580. DOI:10.1111/j.1364-3703.2007.00417.x |
| [36] |
M. Borgers, H. Van den Bossche, M. De Brabander, Am. J. Med. 74 (1983) 2-8. |
| [37] |
I. Fournier, J. Barwicz, P. Tancrède, Biochim. Biophys. Acta BBA-Biomembr. 1373 (1998) 76-86. DOI:10.1016/S0005-2736(98)00083-2 |
 2020, Vol. 31
2020, Vol. 31