Hierarchical self-assembly of amphiphilies plays a significant role in living systems, for example, phospholipids self-assembly to form head to head bi-molecules through H-bonding first, then bi-molecules fused into biological membrances due to their amphiphilic nature of them; deoxynucleotide selfassembly into linear polymeric first order of DNA through H-bonding, then second order double helix structure formed by reverse parallel coiling of two deoxypolynucleotide chains; further specific spatial structures formed by twisting and coiling of double helix structures, also known as supra-helix structures [1]. Inspired by such natural process, chemists and materials scientists have designed and prepared various artificial amphiphilies, such as surfactants, amphiphilic rodcoil molecules, amphiphilic linear polymers and macrocyclic amphiphilies, with hierarchical self-assembly properties [[2]]. For example, Prof. Huang and co-workers reported the efficient preparation of two amphiphilic rhomboids that can subsequently order into various nano-structures [2a].
Among various synthetic amphiphilies, macrocyclic amphiphilies have drawn much attention due to their superiority in selfassembly process [3]. At the same time, a new technology called "coordination-driven self-assembly" is developed and applied to construct discrete coordination macrocyclic compounds (CMCCs) [4]. As there is a wide range of metal acceptors and organic donors that are suitable for this technology, a large number of CMCCs with different shapes and dimensionality, like 2D metallacycles, and 3D metallacages, were successfully prepared by this method directly [5]. Importantly, coordination macrocyclic amphiphilies combined the self-assembly property from amphiphilic nature, the hostguest property from macrocyclic nature, the optical, electrical and magnetic properties from inorganic metal atoms, which enhanced their application in many areas, such as controlled release, cancer therapy, photoelectric materials, and so on [6].
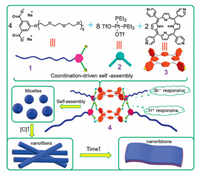
In this paper, we reported the design and synthesis of a discrete 3D amphiphilic metallacage 4 via "coordination driven selfassembly", in which the tetragonal prismatic frameworks contained porphyrin units act as hydrophobic cores and poly(ethylene glycol) (PEG) chains as the hydrophilic tails. The structure of 4 was characterized by 1H NMR, 31P NMR and ESI-TOF-MS. Notably, 4 with its long PEG tails was subsequently ordered into micelles, nanofibers, and nanoribbons, depending on the conditions involved in its self-assembly process (Scheme 1).
|
Download:
|
| Scheme 1. Self-assembly of 1, 2, 3 to give amphiphilic metallacage 4, and 4 further self-assembly into micelles, nanofibers and nanoribbons. | |
As we reported previously, equivalent amount of carboxylatebased ligands and pyridyl-based ligands that assemble with 90 Pt(Ⅱ) acceptors 2 can obtain neutral metallacage [7]. In our current work, the amphiphilic organoplatinum(Ⅱ) metallacage 4 was obtained by stirring water-soluble carboxylate ligand 1, 90° Pt(Ⅱ) acceptor 2, and tetrapyridylporphyrin 3 in a ratio of 4:8:2 in H2O/ acetone (1:4) at 60 ℃ overnight. The isolated product was analyzed by 1H NMR and 31P NMR spectroscopies, which indicates the formation of discrete and high symmetric structure. As shown in Fig. 1, the 31P{1H} NMR spectra of 4 in acetone-d6 show two coupled doublets of equal intensity at about δ -2.87 and δ 2.4 with concomitant 195Pt satellites although only one intense singlet at 7.78 ppm was observed for precusor 2 in the same solvent. This splitting supports the heteroleptic (Pt←N and Pt←O) coordination of the Pt(Ⅱ) centers in the complexes wherein the most shielded peak likely represents the phosphorus nuclei trans to the pyridine ring and the phosphorus nuclei opposite to the carboxylate group are characterized by the relatively less shielded peak [8].

|
Download:
|
| Fig. 1. 31P {1H} NMR spectra (room temperature, 121.4 MHz) of (a) 90° Pt(Ⅱ) acceptor 2 in acetone-d6, (b) metallacage 4 in acetone-d6. | |
In addition, 1H NMR spectra (Fig. S1 in Supporting information) gave more evidence for the formation of coordinated carboxylate and pyridyl moieties: for 4, 9.43 (Hα-Py), 8.52 (Hphenyl), 8.43 (Hpyrrole), 7.75 (Hphenyl), 7.69 (Hβ-py).
Further evidence for the formation of metallacage with the expected stoichiometry was obtained from the electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) data. As shown in Fig. 2, peaks corresponding to metallacages after the loss of eight trifluoromethanesulfonate (OTf) counterions were observed at m/z = 1014.32 for 4. The peaks are isotopically resolved and agree well with their theoretical distributions.

|
Download:
|
| Fig. 2. Calculated (blue) and experimental (red) ESI-TOF-MS spectra of metallacage 4 [M-8OTf]8+. | |
With amphiphilic metallacage 4 in hand, we then investigated its amphiphilic nature. 4 can dissolve in most of the common solvents and corresponding solutions are stable as no precipitations even overnight (Fig. S4 in Supporting information). As shown in Fig. 3a, the metallacage displayed the characteristic Soret band at about 420 nm and Q bands at about 515 nm, 545 nm, and 582 nm. However, the intensity of the peaks sharply decreased in the aqueous solution compared with other organic solutions. This decrease indicated that the metallacages might self-assemble into multi-molecular aggregates in water, so the absorption species decreased. Fluorescence emission spectra of metallacage showed two characteristic bands based on porphyrin at about 650 nm and 720 nm (Fig. 3b). The intensities of the peaks in water were also weaker than in other organic solvents, which consisted with the UV investigation.

|
Download:
|
| Fig. 3. (a) UV–vis absorption spectra of 4 in different solvents. (b) Fluorescence emission 4 in different solvents. | |
Then we investigated the self-assembly behavior of metallacage 4 in water. The critical aggregate concentration (CAC) of metallacage in water was determined by the water surface tension (γ) against the concentration of 4 (C) firstly [9]. As shown in Fig. S5 (Supporting information), the sudden decrease values of g indicated that the CAC of 4 is 1.10×10-6 mol/L. What is more, tyndall effects (Fig. S6 in Supporting information) were also observed in the aqueous solution of 4 (1.20×10-6 mol/L), indicating that metallacage can self-assembly into nanoaggregates in water [10], which agree with the above spectra studies. In addition, the tyndall effects did not occur to the solutions of free ligand 1. This is due to the free ligands are totally hydrophilic and can dissolved in water in mono-molecular state (Fig. S6).
Dynamic light scattering (DLS) experiment performed with a 1.20×10-6 mol/L aqueous solution of 4 over a scattering angle of 90 showed a narrow size distribution (Fig. S7 in Supporting information). The average hydrodynamic diameter (Dh) of 4 was observed to be about 42 nm. In sharp contrast, the Dh values of the free ligand 1 (1.20×10-6 mol/L) is < 5 nm in water, indicating that the free ligand could not form aggregations in water at this concentration, consistent with the results of tyndall effects (Fig. S8 in Supporting information). Then we used transmission electron microscopy (TEM) to investigate the self-assembly behaviors of metallacages in water. As shown in Fig. 4a, when 4 was dissolved in water with a concentration of about 1.20×10-6 mol/L, it selfassembled into micelles, whose average diameter was about 40 nm, larger than the length of the 4 (Fig. S9 in Supporting informaiton), indicating that the micelles self-assembled from several molecules fusing together (Fig. 4c). 4 self-assembled into micelles in water possible due to its larger curvature of the membrane, and membrane with larger curvature could form micelles easily (Fig. S10) [11].

|
Download:
|
| Fig. 4. (a) TEM image of the 4 self-assembly in water. (b) Enlarge TEM image of 4 self-assembly in water. (c) Schematic diagram of 4 self-assembly in water. | |
Inspired by the dynamic nature of coordination bonds [12], we then further investigated the stimuli-responsive property of vesicles self-assembled from 4. When we added 20 equiv. of tetrabutylammonium bromide (Bu4NBr) into the micelles' solution, the micelles were destroyed and transformed into irregular structures. As shown in Fig. S11a (Supporting information), TEM image confirmed the formation of irregular structures after the addition of Bu4NBr. Furthermore, 31P NMR studies give the convinced evidence to support the reversible bromide-responsive disassembly process. As shown in Fig. 5a, after the addition of Bu4NBr to the solution of metallacages in water, the typical signals of metallacages disappeared gradually with the stirring time increased, and the resonance from new Pt-Br complex appeared in the 31P NMR spectra (Fig. 5a, Ⅲ). Moreover, when addition of AgOTf to the above system, the original 31P peaks of metallacages were appeared again (Fig. 5a, IV). The above 31P NMR studies showed that the bromide induced disassemblyand reassemblyof the cubelike core of the 4 further determined the morphologies transform of the assemblies. Besides Br- responsiveness, 4 also show H+ responsive property when self-assembly in water. As shown in Fig. S9b, the micelles transformed into irregular structures after the addition of 20 equiv. of HOTf (Fig. S11b in Supporting informaiton). At the same time, 31P NMR studies also give the convinced evidence to support the H+-responsive disassembly process. After the addition of HOTf to the solution of metallacages in water, the typical signals of metallacages disappeared gradually with the stirring time increased (Fig. 5b).

|
Download:
|
| Fig. 5. (a) 31P{1H} NMR spectra (room temperature, 121.4 MHz, water/CH3CN) of adding Bu4NBr into the solution of 4 with different time: (Ⅰ) pure 4; (Ⅱ) 1 min; (Ⅲ) 1 h; (Ⅳ) AgOTf and the solution of (Ⅲ). (b) 31P{1H} NMR spectra (room temperature, 121.4 MHz, water) of adding HOTf into the solution of 4 with different time: (Ⅰ) fresh prepared; (Ⅱ) 10 min; (Ⅲ) 30 min; (Ⅳ) 60 min; (Ⅴ) 6 h. | |
Sincemetallacage 4has arigid, well-definedcore, thepossibility of higher-order nano-structures resulting from additional intermoleclar interactions was investigated. When the concentration of 4 increased from 1.20 10-6 mol/L to 1.00 10-4 mol/L in water, TEM image revealed that the morphology of the resulting assemblies underwent marked changes. As shown in Fig. 6a, amphiphilic metallacage 4 self-assembled into nanofibers with widths about 15 nm and length about 2 μm.Molecular modeling of 4 revealed a maximum PEG tip-to-tip distance of about 12.5 nm (Fig. S9), in agreement with the observed diameters of single fiber. Further experiments showed that these nanofibers are thermodynamic unstable, as they will transform into nano-ribbons upon the solution standing over the course overnight (Fig. 6b).

|
Download:
|
| Fig. 6. (a) TEM image of 4 self-assembly into nanofibers. (b) TEM image of 4 selfassembly into nanoribbons. | |
In conclusion, a 3D discrete amphiphilic organoplatinum(Ⅱ) metallacage 4 with cube-like cage as the hydrophobic core and ethylene glycol chains as the hydrophilic tail was successfully prepared. It can subsequently self-assemble into well-defined micellesinwater. With theconcentrationof metallacageincreased, the micelles can transform into nanofibers. However, these nanofibers are thermodynamic unstable, they can further transform into nano-ribbons upon the solution standing over the course overnight. This design exploits hierarchical assembly with multiple interactions working in concert to construct complex nanomaterials. The first level of assembly is the spontaneous formation of discrete matallacage 4. The amphiphilic nature of 4 motivates secondary ordering into micelles, which can further assemble by a third intermolecular interaction to give nanofibers and nanoribbons. This unification of coordination driven self-assembly and amphiphilic self-assembly defines a new approach to fabrication of soft materials with fine control over the dimensionality, given potential applications in biosystems and materials science.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21801139), Natural Science Foundation of Jiangsu Province (No. BK20180942), the Natural Science Foundation of Nantong University for High-Level Talent (No. 03083004).
Appendix A. Supplementary dataSupplementary material related to this article can be found, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2019.08.036.
| [1] |
(a) J.W. Bryson, S.F. Betz, H.S. Lu, et al., Science 270 (1995) 935-941; (b) Y. Cao, Y. Duan, L. Han, S. Che, Chem. Commun. 53 (2017) 5641-5644; (c) S.M.F. Vilela, P. Salcedo-Abraira, L. Micheron, et al., Chem. Commun. 54 (2018) 13088-13091; (d) C.W. Chu, B.J. Ravoo, Chem. Commun. 53 (2017) 12450-12453; (e) W. Lin, T.Y. Cen, S.P. Wang, et al., Chin. Chem. Lett. 29 (2018) 1372-1374; (f) S.P. Wang, W. Lin, X. Wang, et al., Nat. Commun. 10 (2019) 1399. |
| [2] |
(a) X. Yan, S. Li, T.R. Cook, et al., J. Am. Chem. Soc. 135 (2013) 14036-14039; (b) H.J. Kim, T. Kim, M. Lee, Acc. Chem. Res. 44 (2011) 72-82; (c) Y. Liu, C. Yu, H. Jin, et al., J. Am. Chem. Soc. 135 (2013) 4765-4770; (d) S. Sun, M. Geng, L. Huang, et al., Chem. Commun. 54 (2018) 13006-13009; (e) A. Sakaguchi, K. Higashiguchi, K. Matsuda, Chem. Commun. 54 (2018) 4298-4301; (f) M. He, L. Chen, B. Jiang, et al., Chin. Chem. Lett. 30 (2019) 131-134; (g) J. Chen, Y. Wang, C. Wang, et al., Chem. Commun. 55 (2019) 6817-6826. |
| [3] |
(a) Z.Q. Cao, Y.C. Wang, A.H. Zou, et al., Chem. Commun. 53 (2017) 8683-8686; (b) K. Jie, Y. Zhou, Y. Yao, F. Huang, Chem. Soc. Rev. 44 (2015) 3568-3587; (c) I. Pisagatti, L. Barbera, G. Gattuso, et al., New J. Chem. 43 (2019) 7628-7635; (d) P.Zhang, E.Paszkiewicz, Q.Wang, etal., Chem.Commun.53 (2017)10528-10531; (e) Y. Zhou, K. Jie, F. Huang, Chem. Commun. 54 (2018) 12856-12859; (f) Y. Chen, S. Sun, D. Lu, et al., Chin. Chem. Lett. 30 (2019) 37-43; (g) Y. Yao, X. Wei, J. Chen, et al., Supramol. Chem. 30 (2018) 610-619; (h) T. Xiao, W. Zhong, L. Zhou, et al., Chin. Chem. Lett. 30 (2019) 31-36. |
| [4] |
(a) M. Fujita, M. Tominaga, A. Hori, B. Therrien, Acc. Chem. Res. 38 (2005) 369-378; (b) C.G. Oliveri, P.A. Ulmann, M.J. Wiester, C.A. Mirkin, Acc. Chem. Res. 41 (2008) 1618-1629; (c) B.H. Northrop, Y.R. Zheng, K.W. Chi, P.J. Stang, Acc. Chem. Res. 42 (2009) 1554-1563; (d) S. De, K. Mahata, M. Schmittel, Chem. Soc. Rev. 39 (2010) 1555-1575; (e) N.N. Adarsh, P. Dastidar, Chem. Soc. Rev. 41 (2012) 3039-3060; (f) L.E. Kreno, K. Leong, O.K. Farha, et al., Chem. Rev. 112 (2012) 1105-1125; (g) T.R. Cook, Y.R. Zheng, P.J. Stang, Chem. Rev. 113 (2013) 734-777; (h) T.R. Cook, P.J. Stang, Chem. Rev. 115 (2015) 7001-7045; (i) C.J. Brown, F.D. Toste, R.G. Bergman, K.N. Raymond, Chem. Rev. 115 (2015) 3012-3035. |
| [5] |
(a) Q.F. Sun, S. Sato, M. Fujita, Nat. Chem. 4 (2012) 330-333; (b) J. Fan, M.L. Saha, B. Song, H. Schonherr, M. Schmittel, J. Am. Chem. Soc. 134 (2012) 150-153; (c) A. Sautter, B.K. Kaletas, D.G. Schmid, et al., J. Am. Chem. Soc. 127 (2005) 6719-6729. |
| [6] |
(a) Y. Inokuma, M. Kawano, M. Fujita, Nat. Chem. 3 (2011) 349-358; (b) X. Yan, S. Li, J.B. Pollock, et al., Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 15585-15590; (c) X. Yan, B. Jiang, T.R. Cook, et al., J. Am. Chem. Soc.135 (2013) 16813-16816; (d) Z.Y. Li, Y. Zhang, C.W. Zhang, et al., J. Am. Chem. Soc.136 (2014) 8577-8589; (e) X. Yan, J.F. Xu, T.R. Cook, et al., Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 8717-8722; (f) M. Yamashina, M.M. Sartin, Y. Sei, et al., J. Am. Chem. Soc. 137 (2015) 9266-9269; (g) B. Roy, A.K. Ghosh, S. Srivastava, P. D'Silva, P.S. Mukherjee, J. Am. Chem. Soc. 137 (2015) 11916-11919. |
| [7] |
(a) Y. Wang, C. Wang, R. Long, et al., Chem. Commun. 55 (2019) 5167-5170; (b) Y. Yao, R. Zhao, Y. Shi, et al., Chem. Commun. 54 (2018) 8068-8071. |
| [8] |
Y. Shi, I. Sanchez-Molina, C. Cao, et al., Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 9390-9395. DOI:10.1073/pnas.1408905111 |
| [9] |
Y. Yao, M. Xue, J. Chen, et al., J. Am. Chem. Soc. 134 (2012) 15712-15715. DOI:10.1021/ja3076617 |
| [10] |
(a) G. Yu, X. Zhou, Z. Zhang, et al., J. Am. Chem. Soc. 134 (2012) 19489-19497; (b) K. Wang, D.S. Guo, X. Wang, Y. Liu, ACS Nano 5 (2011) 2880-2894. |
| [11] |
C. Wang, S. Yin, S. Chen, et al., Angew. Chem. Int. Ed. 47 (2008) 9049-9052. DOI:10.1002/anie.200803361 |
| [12] |
(a) L.J. Chen, G.Z. Zhao, B. Jiang, et al., J. Am. Chem. Soc.136 (2014) 5993-6001; (b) S. Li, J. Huang, T.R. Cook, et al., J. Am. Chem. Soc. 135 (2013) 2084-2087; (c) S. Li, J. Huang, F. Zhou, et al., J. Am. Chem. Soc. 136 (2014) 5908-5911. |
 2020, Vol. 31
2020, Vol. 31 

