b Chongqing Key Laboratory of Natural Product Synthesis and Drug Research, School of Pharmaceutical Sciences, Chongqing University, Chongqing 401331, China;
c Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California San Diego, La Jolla 92093-0934, United States
Since the isolation of the first member in 1909, more than 320 Daphniphyllum alkaloids with a vast range of structural diversity have been isolated from the genus Daphniphyllum that comprises over 34 species of dioecious evergreen trees and shrubs mainly distributed from tropical to subtropical Asia [1-3]. Some of these alkaloids have shown interesting biological activities including anticancer, antioxidation, antiviral, vasorelaxation, nerve growth factor regulation, anti-HIV and anti-platelet activating factor effects [3-5]. Structurally, these triterpenoid alkaloids with highly variable polycyclic skeletons can be categorized into more than 20 different types based on the unusual ring systems [4]. Together, the extensive physiological properties and the intriguing architectural features of these alkaloids has attracted considerable attention from the synthetic chemistry community for decades [3], especially the daphmanidin A-type and the calyciphylline A-type subfamilies. Heathcock et al. made the seminal contributions to elegant biomimetic synthesis of several structural types of nature molecules within Daphniphyllum family [6]. Whilst further synthetic efforts toward these enchanting alkaloids have been reported by Carreira [7], Smith [8, 9], Li [10-12], Yokoshima/ Fukuyama [13], Zhai [14], Paton/Dixon [15], Xu [16] and Gao [17].
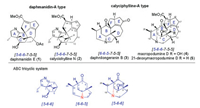
The daphmanidin A-type [18-22] and the calyciphylline A-type [1-3, 10-15, 23-36] Daphniphyllum alkaloids, with significantly different ABC tricyclic frameworks, have characteristic structural backbone consisting of six fused rings [5-6-6-7-5-5] and [6-6-5-7-5-5], respectively (Fig. 1). Calycionhylline N (2), a emblematic daphmanidin A-type Daphniphyllum alkaloid, was isolated by Kobayashi et al. in 2008 [21], including a fused A ring dihydropyrrole and a bicyclo[2.2.2]octane BC ring. It has six contiguous stereocenters, three of which are bridgehead quaternary and two are vicinal quaternary. In addition, macropodumine D (4) and 21-deoxymacropodumine D (5) [37], the unique calyciphylline A-type alkaloids, possess an inimitable [5-6-6-7-5-5] hexacyclic system, containing an exceptional N-C3 linkage and a hydroxyl group at C4, which differ from most members of this type with N-C4 linkage. Along with seven consecutive stereocenters including the vicinal all-carbon quaternary centers, it has led to a surge in synthetic interest recently.
|
Download:
|
| Fig. 1. Typical ABC tricyclic systems of Daphniphyllum alkaloids. | |
The formidable challenge posed by the structural intricacies presented in the ABC tricyclic systems, especially [5-6-6] tricyclic unit, with limited total synthetic reports, intrigues us to develop a unified strategy that would be applied in the synthesis of related congeners bearing the tricyclic ABC ring systems. Herein, we report our routes of asymmetric synthesis of ABC tricyclic framework of calycionhylline N (2) and 21-deoxymacropodumine D (5).
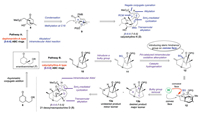
Our synthetic plan is illustrated in Scheme 1, with calyciphylline N (2) and 21-deoxymacropodumine D (5) as exemplary targets. We designed pathway A and B to construct [5-6-6] tricyclic frameworks from enantioenriched ketone 7 with all-carbon quaternary C5, which could be accessed from readily available enone 6 via an asymmetric conjugate addition [38]. For construction of the bicyclo[2.2.2]octanone B/C core 8, we conceived that alkylation/intramolecular Aldol tandem reaction of 7 could forge the rudimentary/Critical bridged bicyclic structure with two bridgehead all-carbon quaternary centers (Scheme 1, pathway A). The formation of A ring could be enabled by the condensation of the primary amine with C1 carbonyl group. Furthermore, calyciohylline N (2) could be assembled from the key tricyclic [5-6-6] intermediate 9 via sequential reactions that includes methylation at C18, ring closing metathesis (RCM) reaction [39, 40], Nagata conjugate cyanation [41-44], SmI2-mediated cyclization [45] and transannular alkylation.
|
Download:
|
| Scheme 1. An initial strategy for constructing ABC tricycle of calyciphylline N (2) and 21-deoxymacropodumine D (5). PG = protecting group. BG = bulky group. | |
In our initial attempt [36], we obtained the desired key intermediate 13b with tricyclic [5-6-6] structure in low yield and unsatisfied stereoselectivity at C18. According to our analysis, the hydrogenation of double bond at C18, occurring dominantly from convex face of the alkene substrate, might lead to the poor stereoselectivity. We anticipated to derive stereochemical control from introducing a sterically bulky and easily removable group at C2 of bicyclic intermediate 10 (Scheme 1, pathway B). Formation of the desired ABC [5-6-6] tricyclic core 13b would be achieved through Pd-catalyzed intramolecular oxidative alkylation [46-48], catalytic hydrogenation from concave face of the alkene and bulky group removal in sequential. In this event, the steric hindrance of the bulky group on convex face could promote the catalytic hydrogenation occurring from concave face of the tricyclic alkene 12. Additionally, the DEF rings of 21-deoxymacropodumine D (5) could be built via intramolecular Aldol reaction, SmI2-mediated cyclization and transannular alkylation, which was similar with the strategy of synthesis of calyciohylline N (2).
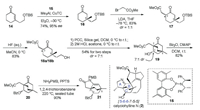
Last year, we synthesized the ABCF tetracyclic framework of Daphniphyllum alkaloid calyciphylline N (2) by serving the inter/ intramolecular aldol sequence and the stereoselective Nagata conjugate cyanation as the key reactions [22]. Here, we plan to use an alternative strategy to assemble the challenge of constructing the bicyclo[2.2.2]octane BC ring with two bridegehead all-carbon quaternary centers. Our synthesis of ABC [5-6-6] tricyclic unit of calyciphylline N (2) commenced with the copper-catalyzed asymmetric conjugate addition developed by Vuagnoux-d'Augustin and Alexakis [38], affording an enantioenriched ketone intermediate 16 with C5 all-carbon quaternary center in 74% yield and excellent enantioselectivity up to 95% ee (Scheme 2). Whereafter, the resultant ketone 16 was subjected to alkylation with methyl 2-bromoacetate in the presence of LDA in THF at -78 ℃, affording the desired product 17 as a mixture of two diastereoisomers (1:1 ratio) in 83% combined yield. After the deprotection of TBS group, compound 18 was then transformed into the key bicyclo[2.2.2]octanone B/C core 19 by sequential oxidation of resulting alcohol with PCC and acid-mediated intramolecular Aldol reaction in 54% yield for two steps with high stereoselectivity at C7 (7:1 dr). It is worthwhile to mention that this acid-mediated intramolecular Aldol type cyclization performed with high endo selectivity. With the desired bicyclic key intermediate 19 in hand, the late stage was set for the key transformation to construct the critical ABC [5-6-6] tricyclic unit. Protection of the secondary alcohol in 19 followed by thermal condensation delivered the A/B/C tricycle 21 in the presence of PMBNH2 and PPTS with high yield. Thus, the A/B/C tricyclic framework of calyciphylline N (2), which comprises bicyclo[2.2.2]octanone and three quaternary stereocenters, was synthesized in 7 steps from the known unsaturated ketone 14.
|
Download:
|
| Scheme 2. Preparation of ABC tricyclic [5-6-6] system of calyciohylline N (2). | |
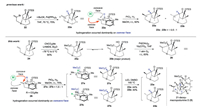
Then, we turned our attention to investigate the synthesis of ABC tricyclic [5-6-6] unit of 21-deoxymacropodumine D (5). Since the strategy of Pd-catalyzed enolate α-alkylation lead to ABC tricyclic [5-6-6] framework 23a with unacceptable yield and disappointing approximate 0.5:1 ratio of diastereomers (Scheme 3), we focused on improving the stereoselectivity at C18 via introducing a suitable bulky group at C2 (as previously mention in retro synthetic analysis, Scheme 1, pathway B).
|
Download:
|
| Scheme 3. Improved preparation of ABC tricyclic [5-6-6] system of 21-deoxymacropodumine D (5). | |
Our optimized synthesis commenced with bicyclic ketone 24, which we previously utilized for the synthetic studies of 21- deoxymacropodumine D (5) via radical cyclization strategy [38]. Regioselective deprotonation of 24 with LiHMDS followed by quenching of the anion with Mander's reagent provided enol 25b as the major product. To implement the Pd-catalyzed intramolecular oxidative alkylation, enol 25b was treated with Pd(OAc)2 and Yb(OTf)3 in THF under O2 atmosphere [46-48], generating the C2- C18 ligated compound 26 in 83% yield. Catalytic hydrogenation of the double bond in 26 with PtO2 in MeOH afforded an inseparable mixture of two diastereoisomers in a 1.5:1 ratio determined by the 1H NMR spectrum. The relative stereochemistry of 27a and 27b was determined by comparing the NMR spectrum of the Krapcho decarboxylation products 23a and 23b. The relative stereochemistry of compounds 23a and 23b was determined by the NOE correlations, which indicates that the major isomer is our desired product 23a. Therefore, the ABC tricyclic unit of 21- deoxymacropodumine D (5) with five consecutive stereogenic centers including an all-carbon quaternary center was successfully synthesized, which improves the stereoselectivity at C18 from 0.5:1 to acceptable 1.5:1 ratio of diastereomers.
In summary, we have established an efficient synthetic route of ABC [5-6-6] ring systems, which is a potential useful method in the total synthesis of daphmanidin A-type and calyciphylline A-type alkaloids. The asymmetric synthesis of ABC [5-6-6] tricyclic frame of calyciohylline N (2) via acid-mediated intramolecular Aldol reaction to construct the desired bicyclo[2.2.2]octane core and a thermal condensation to afford the ABC tricyclic framework was achieved. Meanwhile, the improved synthesis of ABC [5-6-6] tricyclic system of 21-deoxymacropodumine D (5) was also achieved. It features a Pd-catalyzed intramolecular oxidative alkylation as the key reaction to construct the desired bowlshaped tricyclic core and promising stereoselectivity at C18 (1.5:1 ratio of diastereomers) by introducing a steric repulsion on convex face. Inspired by these initial studies, the total synthesis of daphmanidin A-type and calyciphylline A-type alkaloids are undergoing in our lab.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21502011), the Fundamental Research Funds for the Central Universities and the Chongqing Science & Technology Commission Project (No. cstc2016jcyjA0168).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.08.033.
| [1] |
J. Kobayashi, T. Kubota, Nat. Prod. Rep. 26 (2009) 936-962. DOI:10.1039/b813006j |
| [2] |
B. Kang, P. Jakubec, D.J. Dixon, Nat. Prod. Rep. 31 (2014) 550-562. DOI:10.1039/C3NP70115H |
| [3] |
A.K. Chattopadhyay, S. Hanessian, Chem. Rev. 117 (2017) 4104-4146. DOI:10.1021/acs.chemrev.6b00412 |
| [4] |
H.F. Wu, X.P. Zhang, L.S. Ding, et al., Planta Med. 79 (2013) 1589-1598. DOI:10.1055/s-0033-1351024 |
| [5] |
J.B. Xu, H. Zhang, L.S. Gan, et al., J. Am. Chem. Soc. 136 (2014) 7631-7633. DOI:10.1021/ja503995b |
| [6] |
C.H. Heathcock, Angew. Chem. Int. Ed. 31 (1992) 665-804. DOI:10.1002/anie.199206653 |
| [7] |
M.E. Weiss, E.M. Carreira, Angew. Chem. Int. Ed. 50 (2011) 11501-11505. DOI:10.1002/anie.201104681 |
| [8] |
A. Shvartsbart, A.B. Smith III, J. Am. Chem. Soc. 136 (2014) 870-873. DOI:10.1021/ja411539w |
| [9] |
A. Shvartsbart, A.B. Smith III, J. Am. Chem. Soc. 137 (2015) 3510-3519. DOI:10.1021/ja503899t |
| [10] |
Z.Y. Lu, L. Yong, J. Deng, A. Li, Nat. Chem. 5 (2013) 679-684. DOI:10.1038/nchem.1694 |
| [11] |
J. Li, W.H. Zhang, F. Zhang, Y. Chen, A. Li, J. Am. Chem. Soc. 139 (2017) 14893-14896. DOI:10.1021/jacs.7b09186 |
| [12] |
Y. Chen, W.H. Zhang, L. Ren, J. Li, A. Li, Angew. Chem. Int. Ed. 57 (2018) 952-956. DOI:10.1002/anie.201711482 |
| [13] |
R. Yamada, Y. Adachi, S. Yokoshima, T. Fukuyama, Angew. Chem. Int. Ed. 55 (2016) 6067-6070. DOI:10.1002/anie.201601958 |
| [14] |
X.M. Chen, H.J. Zhang, X.K. Yang, et al., Angew. Chem. Int. Ed. 57 (2018) 947-951. DOI:10.1002/anie.201709762 |
| [15] |
H.Y. Shi, I.N. Michaelides, B. Darses, et al., J. Am. Chem. Soc. 139 (2017) 17755-17758. DOI:10.1021/jacs.7b10956 |
| [16] |
Y. Chen, J. Hu, L.D. Guo, et al., Angew. Chem. Int. Ed. 58 (2019) 7390-7394. DOI:10.1002/anie.201902908 |
| [17] |
J. Zhong, K. Chen, Y. Qiu, H. He, S. Gao, Org. Lett. 21 (2019) 3741-3745. DOI:10.1021/acs.orglett.9b01184 |
| [18] |
J. Kobayashi, S. Ueno, H. Morita, J. Org. Chem. 67 (2002) 6546-6549. DOI:10.1021/jo0258204 |
| [19] |
T. Kubota, Y. Matsuno, H. Morita, et al., Tetrahedron 62 (2006) 4743-4748. DOI:10.1016/j.tet.2006.03.040 |
| [20] |
H. Morita, N. Ishioka, H. Takatsu, T. Iizuka, J. Kobayashi, J. Nat. Prod. 69 (2006) 418-420. DOI:10.1021/np0503799 |
| [21] |
H. Yahata, T. Kubota, J. Kobayashi, J. Nat. Prod. 72 (2009) 148-151. DOI:10.1021/np800515s |
| [22] |
Y.F. Li, Q.Y. Dong, Q.X. Xie, et al., Org. Lett. 20 (2018) 5053-5057. DOI:10.1021/acs.orglett.8b02202 |
| [23] |
B.W. Fang, H.J. Zheng, C.G. Zhao, et al., J. Org. Chem. 77 (2012) 8367-8373. DOI:10.1021/jo301533f |
| [24] |
H.L. Li, J.Y. Zheng, S.Y. Su, et al., Chem. -Asian J. 7 (2012) 2519-2522. DOI:10.1002/asia.201200625 |
| [25] |
H.L. Li, Y.C. Qiu, C.G. Zhao, et al., Chem. -Asian J. 9 (2014) 1274-1277. DOI:10.1002/asia.201400002 |
| [26] |
W. Wang, G.P. Li, S.F. Wang, Z.F. Shi, X.P. Cao, Chem. -Asian J. 10 (2015) 377-382. DOI:10.1002/asia.201403152 |
| [27] |
Y.M. Yao, G.X. Liang, Org. Lett. 14 (2012) 5499-5501. DOI:10.1021/ol3026395 |
| [28] |
X.C. Xiong, Y. Li, Z.Y. Lu, et al., Chem. Commun. 50 (2014) 5294-5297. DOI:10.1039/c3cc47873d |
| [29] |
L. Wang, C. Xu, L. Chen, X.J. Hao, D.Z.G. Wang, Org. Lett. 16 (2014) 1076-1079. DOI:10.1021/ol403609c |
| [30] |
A.A. Ibrahim, A.N. Golonka, A.M. Lopez, J.L. Stockdill, Org. Lett. 16 (2014) 1072-1075. DOI:10.1021/ol4034868 |
| [31] |
D. Ma, H. Cheng, C.M. Huang, L. Xu, Tetrahedron Lett. 56 (2015) 2492-2495. DOI:10.1016/j.tetlet.2015.03.097 |
| [32] |
J.L. Stockdill, A.M. Lopez, A.A. Ibrahim, Tetrahedron Lett. 56 (2015) 3503-3506. DOI:10.1016/j.tetlet.2015.01.121 |
| [33] |
J.J. Guo, Y. Li, B. Cheng, et al., Chem. -Asian J. 10 (2015) 865-868. DOI:10.1002/asia.201403061 |
| [34] |
G. Coussanes, J. Bonjoch, Org. Lett. 19 (2017) 878-881. DOI:10.1021/acs.orglett.7b00035 |
| [35] |
H. Shao, W. Bao, Z.R. Jing, et al., Org. Lett. 19 (2017) 4648-4651. DOI:10.1021/acs.orglett.7b02274 |
| [36] |
X.F. Mo, Y.F. Li, M.H. Sun, et al., Tetrahedron Lett. 59 (2018) 1999-2004. DOI:10.1016/j.tetlet.2018.04.019 |
| [37] |
H. Zhang, D.D. Zhang, J.Y. Li, S.L. Shyaula, J. Li, J.M. Yue, RSC Adv. 6 (2016) 44402-44409. DOI:10.1039/C6RA06420E |
| [38] |
M. Vuagnoux-d' Augustin, A. Alexakis, Chem. -Eur. J. 13 (2007) 9647-9662. DOI:10.1002/chem.200701001 |
| [39] |
H. Abe, A. Sato, T. Kobayashi, H. Ito, Org. Lett. 15 (2013) 1298-1301. DOI:10.1021/ol400228v |
| [40] |
N. Galy, D. Moraleda, M. Santelli, Tetrahedron lett. 50 (2009) 5238-5240. DOI:10.1016/j.tetlet.2009.07.033 |
| [41] |
W. Nagata, M. Yoshioka, Org. React. 25 (1977) 255-476. |
| [42] |
S.J. Min, S.J. Danishefsky, Angew. Chem. Int. Ed. 46 (2007) 2199-2202. DOI:10.1002/anie.200605058 |
| [43] |
S.K. Murphy, M.S. Zeng, S.B. Herzon, Science 356 (2017) 956-959. DOI:10.1126/science.aan0003 |
| [44] |
M. Zeng, S.K. Murphy, S.B. Herzon, J. Am. Chem. Soc. 139 (2017) 16377-16388. DOI:10.1021/jacs.7b09869 |
| [45] |
G.A. Molander, J.C. Mcwilliams, B.C. Noll, J. Am. Chem. Soc. 119 (1997) 1265-1276. DOI:10.1021/ja963195c |
| [46] |
M. Saeki, M. Toyota, Tetrahedron Lett. 51 (2010) 4620-4622. DOI:10.1016/j.tetlet.2010.06.114 |
| [47] |
A. Hibi, M. Toyota, Tetrahedron Lett. 50 (2009) 4888-4891. DOI:10.1016/j.tetlet.2009.06.052 |
| [48] |
L.R. Kung, C.H. Tu, K.S. Shia, H.J. Liu, Chem. Commun. (2003) 2490-2491. |
 2020, Vol. 31
2020, Vol. 31 

