b Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, East China Normal University, Shanghai 200062, China
Owning to their prevalence in a wide range of natural products and pharmaceutically important molecules [1-6], as well as challenge associated with the three dimensional structure control imposed by a spiro-ring fusion [7-9], spirocyclic oxindoles have evoked immense interest from the synthetic standpoint recently [10-15]. To further modulate the biological properties of spirocyclic oxindoles, an effective strategy is to incorporate different-sized heterocycles into C3 position to bring about improved biological activities [16-21]. On the other hand, sulfur-containing heterocycles such as (tetrahydro)thiophene and (tetrahydro)thiopyran are widely distributed in a variety of biologically active natural products and marketed drugs [22-26]. About one-tenth of the top 200 most-prescribed drugs in 2016 contain sulfur heterocycles [27]. As is reasonably expected, exploiting new strategies that can integrate sulfur heterocycles with oxindoles will be potentially beneficial for the drug discovery [28-31]. For example, guided by this 'integration' idea, Sheng and co-workers validated that the spirooxindole fused with tetrahydrothiopyran scaffold is a new class of p53-MDM2 protein-protein interaction inhibitor with good antitumor activity [31]. However, comparing with the established strategies focusing on incorporating N- or O-heterocycles [10-14], progress towards the fusion of sulfur heterocycles with oxindoles is slow [31-43], since the seminal contribution from Xiao group in 2012 [32]. As for the preparation of spirooxindoles bearing sulfurcontaining six-membered rings, limited examples based on 3- sulfur substituted oxindoles [31, 40, 41] and methyleneindolinones [42, 43] via organocatalysis or base catalysis have been disclosed, to construct the spiro[indoline-3, 2'-thiopyran] and spiro[indoline- 3, 3' theless, there is still high demand for developing new catalytic methods for preparing spirooxindoles with novel sulfur-substituted heterocycles starting from easily available substrates.
As a well-established strategy for preparing versatile cyclic compounds, the catalytic cycloaddition with donor-acceptor (D-A) cyclopropanes have attracted extensive attention [44-46]. To achieve the satisfactory control of both reactivity and stereoselectivities, doubly activated cyclopropane-1, 1-dicarboxylates and analogous diketones are often used. In accordance with the aim to synthesize spirocyclic oxindoles, the employment of easily available spirocyclopropyl oxindoles constitutes a convenient way [3, 47-48]. Nevertheless, although Carreira and colleagues first showed the promise by MgI2-catalyzed [3 + 2] cycloaddition reaction with imines in 1999 [49], the potential of such monoactivated D-A cyclopropanes is largely undeveloped [47], which might be attributed to the relatively low reactivity. In view of this, coupled with our interest in oxindole chemistry [11, 48, 50-53], we found that by installing an electron-withdrawing diethoxyphosphoryl group on the nitrogen atom can effectively activate spirocyclopropyl oxindoles recently, enabling the highly stereoselective [3 + 3] cycloaddition with nitrones [50]. Taking advantage of this activation strategy, we also noticed that the [3 + 3] annulation of 2 with cheap 1, 4-di-thiane-2, 5-diol 1 [54-57] worked smoothly via Lewis acid catalysis, offering a new way for delivering structurally novel spiro[indoline-3, 4'-thiopyran]-2-ones 3 (Scheme 1c) [50]. Herein, the details of the method including effects of reaction parameters and the substrate scope are descried in full length.

|
Download:
|
| Scheme 1. Approaches for the synthesis of sulfur-containing six-membered ring fused spirooxindoles. | |
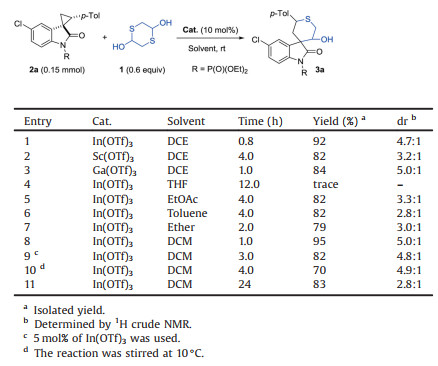
Following our initially reported result [50], we began the investigation of twelve different metal salts by evaluating the annulation of N-diethoxyphosphoryl spirocyclopropyl oxindoles 2a with 1 under the reported condition (10 mol% of metal catalyst, 1, 2-dichloroethane (DCE) as solvent at room temperature) (Table 1). Among them (for full details, please see Table S1 in Supporting information), Mg(OTf)2, Ni(OTf)2, Cu(OTf)2, Zn(OTf)2, La(OTf)3, Y(OTf)3, Er(OTf)3, Yb(OTf)3, Bi(OTf)3 did not work at all, and only In(OTf)3, Sc(OTf)3 and Ga(OTf)3 could promote the [3 + 3] annulation, affording the desired product 3a with moderate to good diastereoselectivities (entries 1–3). Different from known examples whereas Sc(OTf)3 behaves superior for this type of cycloaddition [56, 57], In(OTf)3 gives better result in terms of efficiency and diastereoselectivity in our case (entry 1 vs. 2). Encouraged by this, we further evaluated the solvent effect with In (OTf)3 as the optimal catalyst. It turns out that dichloromethane (DCM) was the best choice and product 3a can be isolated with excellent yield and 5:1 dr (entries 1, 4–8) within only 1 h. However, attempts to further decreasing the catalyst loading or lowering reaction temperature failed by the observation of lower yields (entries 9 and 10). During the condition optimization, we also noticed that the ratio of diastereoselectivity decreased gradually as the reaction went on and no changed (dr = 2.8:1) was observed after 24 h (entry 11), which might be caused by the conversion of kinetic product to thermodynamic one, and indicated that the reaction should be quenched in time in order to prohibiting the undesired epimerization. Therefore, the optimized condition entailed the utilization of 2 (0.15 mmol), 1 (0.09 mmol), In(OTf)3 (10 mol%) as catalyst in DCM at room temperature and the typical procedure of the [3 + 3] cyclization reaction was as follow: to a Schlenk tube was sequentially added In(OTf)3 (8.4 mg, 0.015 mmol, 10 mol%), oxindole 2 (0.15 mmol), 1, 4-dithiane-2, 5-diol 1 (0.09 mmol) and anhydrous CH2Cl2 (1.5 mL). After the resulting solution was stirred at 25 ℃ for the indicated time, the residue was rapidly passed through a glass funnel with a thin layer (5 mm) of silica gel (100 mesh), eluted with CH2Cl2, and concentrated under reduced pressure. To determine the diastereoselectivity of product, the residue was first dissolved in CDCl3, and took some samples for the determination of diastereoselectivity by NMR analysis. Then the sample for analysis and the rest of the product were recombined for column chromatographic purification using petroleum ether/EtOAc (1/1, v/v) as the eluent to afford the desired products 3.
|
|
Table 1 Reaction condition optimization. |
|
|
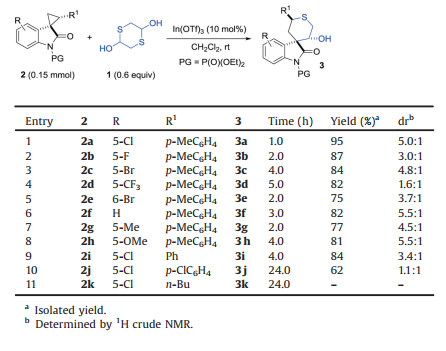
Table 2 Substrate scope evaluation. |
As shown in Fig. 1, the relative configuration of major isomer of 3a (CCDC-1942645) and one of the minor isomer of 3a (CCDC-1890812) were unambitiously confirmed by X-ray crystallographic analysis, which identified that in both cases the hydroxyl and the p-MeC6H4 group are in a trans-configuration, while the configuration on the C3 position of oxindole portion is different.

|
Download:
|
| Fig. 1. ORTEP plot of the crystal structure of 3a. | |
It should be noted that the current activation strategy relied on the use of electron-withdrawing diethoxyphosphoryl group to activate spirocyclopropyl oxindole. As shown by control experiments, the replacement of diethoxyphosphoryl group to methyl or benzyl group, or electron-withdrawing acetyl (Ac), t-butyloxy carbonyl (Boc) and p-toluenesulfonyl (Ts) group, no reaction took place at all (Scheme 2). These results suggest that the N-diethoxyphosphoryl group was very essential for achieving the observed reactivity.

|
Download:
|
| Scheme 2. The dramatic effect of diethoxyphosphoryl group. | |
With the optimal condition in hand, the substrate scope was then evaluated. As shown in Table 2, spirocyclopropyl oxindole 2 bearing both electron-withdrawing and electron-donating substituents in different positions of oxindole ring had negligible effect on the reactivity, and the reaction could finish in five hours or less, enabling the isolation of the desired product 3b-3 h with 75%– 87% yield (entries 2–8). However, a dramatic electronic effect of the substituents on the diastereoselectivity of the reaction was observed. Generally speaking, a slightly higher dr value could be obtained for the substrates bearing electron-donating groups than the ones with electron-withdrawing substituents (entries 6–8 vs. 1–5). Next, the electronic effect of aromatic substituents on cyclopropane part of 2 was examined. Regrettably, we noticed that although the annulation products 3i-3 j can still be obtained with 62%–84% yield, both the reactivity and diastereoselectivity were dramatically decreased in the presence of electron-withdrawing groups such as chloro atom, indicating the very sensitivity of the electronic effect in this position (entries 9 and 10). Unfortunately, when the aromatic group on the cyclopropane part was replaced by an aliphatic butyl group, no reaction took place at all (entry 11).
Inspired by our previous success that chiral Lewis acid Ni(Ⅱ) catalyst could promote the highly enantioselective [3 + 3] annulation of 3-spirooxindole with nitrones via kinetic resolution strategy [50], the asymmetric version of the current [3 + 3] reaction was evaluated. Preliminary attempts indicated the utilization of simple Pybox ligand enabled the promising result. As shown in Eq. (1) (Scheme 3), under the catalysis of 10 mol% of (S, S)-Ph-Pybox 4/In(OTf)3, the annulation of 1 worked slightly selectively with (1S, 2R)-3a, affording the desired chiral product 3a with 48% yield and 20% ee, albeit with lower diastereoselectivity (1.6:1) comparing with the racemic version (entry 8, Table 1). Furthermore, we tested the reaction starting from enantioenriched substrate and the chirality of cyclopropane 2a can be largely transferred to the major isomer of the cycloadduct 3a during the annulation process (Eq. (2)).

|
Download:
|
| Scheme 3. Asymmetric variant evaluation. | |
In summary, we have demonstrated that easily synthesized spirocyclopropyl oxindoles, activated by electron-withdrawing diethyoxyphosphoryl group, can be viewed as a very useful synthon for participating the [3 + 3] annulation reaction with 1, 4- di-thiane-2, 5-diol under Lewis acid catalysis. This method enabled the preparation of structurally novel spiro[indoline-3, 4'-thiopyran]-2-ones bearing (tetrahydro)thiopyran skeleton under very mild condition for the first time, with excellent yield and moderate to good diastereoselectivities. Further studies on the asymmetric version as well as the biological activities evaluation of these sulfur-containing spirocyclic products are in progress in our laboratories.
AcknowledgmentsWe thank the financial support from the National Natural Science Foundation of China (No. 81660576), the Project of Guizhou Province Qian Ke He GZ[2015]4001 and Guizhou Discipline Construction Project (No. GNYL[2017]008).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.08.018.
| [1] |
C.V. Galliford, K.A. Scheidt, Angew. Chem. Int. Ed. 46 (2007) 8748-8758. DOI:10.1002/anie.200701342 |
| [2] |
H. Lin, S.J. Danishefsky, Angew. Chem. Int. Ed. 42 (2003) 36-51. DOI:10.1002/anie.200390048 |
| [3] |
C. Marti, E.M. Carreira, Eur. J. Org. Chem. (2003) 2209-2219. |
| [4] |
B.M. Trost, M.K. Brennan, Synthesis (2009) 3003-3025. |
| [5] |
S. Peddibhotla, Curr. Bioact. Compd. 5 (2009) 20-38. DOI:10.2174/157340709787580900 |
| [6] |
N. Ye, H. Chen, E.A. Wold, P.D. Shi, J. Zhou, ACS Infect. Dis. 2 (2016) 382-392. DOI:10.1021/acsinfecdis.6b00041 |
| [7] |
E.M. Carreira, T.C. Fessard, Chem. Rev. 114 (2014) 8257-8322. DOI:10.1021/cr500127b |
| [8] |
J.H. Xie, Q.L. Zhou, Acta Chim. Sin. 72 (2014) 778-797. DOI:10.6023/A14050364 |
| [9] |
A.S. Ding, M. Meazza, H. Guo, J.W. Yang, R. Rios, Chem. Soc. Rev. 47 (2018) 5946-5996. DOI:10.1039/C6CS00825A |
| [10] |
Z.Y. Cao, F. Zhou, J. Zhou, Acc. Chem. Res. 51 (2018) 1443-1454. DOI:10.1021/acs.accounts.8b00097 |
| [11] |
F. Zhou, Y.L. Liu, J. Zhou, Adv. Synth. Catal. 352 (2010) 1381-1407. DOI:10.1002/adsc.201000161 |
| [12] |
H. Wang, R. Wang, Adv. Synth. Catal. 355 (2013) 1023-1052. DOI:10.1002/adsc.201200808 |
| [13] |
N.R. Ball-Jones, J.J. Badillo, A.K. Franz, Org. Biomol. Chem. 10 (2012) 5165-5181. DOI:10.1039/c2ob25184a |
| [14] |
D.J. Cheng, Y. Ishihara, B. Tan, C.F. Barbas III, ACS Catal. 4 (2014) 743-762. DOI:10.1021/cs401172r |
| [15] |
M. Meazza, R. Rios, Chem. -Eur. J. 22 (2016) 9923-9928. DOI:10.1002/chem.201601893 |
| [16] |
J. Yang, X.W. Liu, D.D. Wang, et al., Tetrahedron 72 (2016) 8523-8536. DOI:10.1016/j.tet.2016.10.050 |
| [17] |
K. Parthasarathy, C. Praveen, J.C. Jeyaveeran, A.A.M. Prince, Bioorg. Med. Chem. Lett. 24 (2016) 4310-4317. DOI:10.1016/j.bmc.2016.07.022 |
| [18] |
S.D. Lotesta, A.P. Marcus, Y.J. Zheng, et al., Bioorg. Med. Chem. 24 (2016) 1384-1391. DOI:10.1016/j.bmc.2016.02.014 |
| [19] |
Y. Arun, K. Saranraj, C. Balachandran, P.T. Perumal, Eur. J. Med. Chem. 74 (2014) 50-64. DOI:10.1016/j.ejmech.2013.12.027 |
| [20] |
S. Haddad, S. Boudriga, T.N. Akhaja, et al., New J. Chem. 39 (2015) 520-528. DOI:10.1039/C4NJ01008F |
| [21] |
M. Gicquel, C. Gomez, M.C.G.A. lvarez, et al., J. Med. Chem. 61 (2018) 9386-9392. DOI:10.1021/acs.jmedchem.8b01137 |
| [22] |
M.H. Feng, B. Tang, S.H. Liang, X.F. Jiang, Curr. Top. Med. Chem. 16 (2016) 1200-1216. DOI:10.2174/1568026615666150915111741 |
| [23] |
J.S. Yu, H.M. Huang, P.G. Ding, et al., ACS Catal. 6 (2016) 5319-5344. DOI:10.1021/acscatal.6b01496 |
| [24] |
P. Chauhan, S. Mahajan, D. Enders, Chem. Rev. 114 (2014) 8807-8864. DOI:10.1021/cr500235v |
| [25] |
B.R. Beno, K.S. Yeung, M.D. Bartberger, L.D. Pennington, N.A. Meanwell, J. Med. Chem. 58 (2015) 4383-4438. DOI:10.1021/jm501853m |
| [26] |
P. Przybylski, K. Pyta-Klich, K. Pyta, A. Janas, Tetrahedron 74 (2018) 6335-6365. DOI:10.1016/j.tet.2018.09.022 |
| [27] |
M.H. Qureshi, D.T. Smith, M.D. Delost, J.T. Njarðarson, Top 200 Pharmaceutical Products by Prescriptions in 2016, (2018). (accessed Oct., 2018) https://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/2016Top200PharmaceuticalPrescriptionSalesPosterLowResV2.pdf.
|
| [28] |
P. Eastwood, J. González, E. Gómez, et al., Bioorg. Med. Chem. Lett. 21 (2011) 5270-5273. DOI:10.1016/j.bmcl.2011.07.033 |
| [29] |
A. Bertamino, M. Soprano, S. Musella, et al., J. Med. Chem. 56 (2013) 5407-5421. DOI:10.1021/jm400311n |
| [30] |
V. Vintonyak, K. Warburg, H. Kruse, et al., Angew. Chem. Int. Ed. 49 (2010) 5902-5905. DOI:10.1002/anie.201002138 |
| [31] |
S.Z. Wang, Y. Jiang, S.C. Wu, et al., Org. Lett. 18 (2016) 1028-1031. DOI:10.1021/acs.orglett.6b00155 |
| [32] |
S. Duan, Y. Li, Y.Y. Liu, et al., Chem. Commun. 48 (2012) 5160-5162. DOI:10.1039/c2cc30931a |
| [33] |
Y.M. Huang, C.W. Zheng, Z. Chai, G. Zhao, Adv. Synth. Catal. 356 (2014) 579-583. DOI:10.1002/adsc.201300833 |
| [34] |
P.F. Zhou, Y.F. Cai, L.L. Lin, et al., Adv. Synth. Catal. 357 (2015) 695-700. DOI:10.1002/adsc.201400964 |
| [35] |
P. Cheng, W.G. Guo, P. Chen, et al., Chem. Commun. 52 (2016) 3418-3421. DOI:10.1039/C5CC10292H |
| [36] |
B.X. Feng, J.D. Yang, J. Li, X. Li, Tetrahedron Lett. 57 (2016) 3457-3461. DOI:10.1016/j.tetlet.2016.06.084 |
| [37] |
Y.Y. Gui, J. Yang, L.W. Qi, et al., Org. Biomol. Chem. 13 (2015) 6371-6379. DOI:10.1039/C5OB00774G |
| [38] |
G.L. Xiao, T.T. Chen, C.Q. Ma, D. Xing, W.H. Hu, Org. Lett. 20 (2018) 4531-4535. DOI:10.1021/acs.orglett.8b01833 |
| [39] |
S.V. Kumar, P. Prasanna, S. Perumal, Tetrahedron Lett. 54 (2013) 6651-6655. DOI:10.1016/j.tetlet.2013.09.123 |
| [40] |
S.Z. Wang, Z.J. Guo, S.Q. Chen, et al., Chem. -Eur. J. 24 (2018) 62-66. DOI:10.1002/chem.201703837 |
| [41] |
S.Z. Wang, S.Q. Chen, Z.J. Guo, et al., Org. Biomol. Chem. 16 (2018) 625-634. DOI:10.1039/C7OB02726E |
| [42] |
T. Arai, T. Miyazaki, H. Ogawa, H. Masu, Org. Lett. 18 (2016) 5824-5827. DOI:10.1021/acs.orglett.6b02783 |
| [43] |
Z. Sun, S.S. Tian, S.L. Li, et al., Tetrahedron Lett. 58 (2017) 3401-3405. DOI:10.1016/j.tetlet.2017.07.033 |
| [44] |
H.K. Grover, M.R. Emmett, M.A. Kerr, Org. Biomol. Chem. 13 (2015) 655-671. DOI:10.1039/C4OB02117G |
| [45] |
T.F. Schneider, J. Kaschel, D.B. Werz, Angew. Chem.Int.Ed. 53 (2014) 5504-5523. DOI:10.1002/anie.201309886 |
| [46] |
(a) S. Liao, X.L. Sun, Y. Tang, Acc. Chem. Res. 47 (2014) 2260-2272; (b) G. Sathishkannan, K. Srinivasan, Chem. Commun. 50 (2014) 4062-4064; (c) X. Fang, J. Li, H.Y. Tao, C.J. Wang, Org. Lett. 15 (2013) 5554-5557. |
| [47] |
Z.Y. Cao, J. Zhou, Org. Chem. Front. 2 (2015) 849-858. DOI:10.1039/C5QO00092K |
| [48] |
Z.Y. Cao, X.M. Wang, C. Tan, et al., J. Am. Chem. Soc. 135 (2013) 8197-8200. DOI:10.1021/ja4040895 |
| [49] |
P.B. Alper, C. Meyers, A. Lerchner, D.R. Siegel, E.M. Carreira, Angew. Chem. Int. Ed. 38 (1999) 3186-3189. DOI:10.1002/(SICI)1521-3773(19991102)38:21<3186::AID-ANIE3186>3.0.CO;2-E |
| [50] |
P.W. Xu, J. Liu, L. Shen, et al., Nat. Commun. 8 (2017) 1619. DOI:10.1038/s41467-017-01451-1 |
| [51] |
F.M. Liao, Z.Y. Cao, J.S. Yu, J. Zhou, Angew. Chem. Int. Ed. 56 (2017) 2459-2463. DOI:10.1002/anie.201611625 |
| [52] |
J.S. Yu, F.M. Liao, W.M. Gao, et al., Angew. Chem. Int. Ed. 54 (2015) 7381-7385. DOI:10.1002/anie.201501747 |
| [53] |
X.P. Yin, X.P. Zeng, Y.L. Liu, et al., Angew. Chem. Int. Ed. 53 (2014) 13740-13745. DOI:10.1002/anie.201407677 |
| [54] |
F. Zamberlan, A. Fantinati, C. Trapella, Eur. J. Org. Chem. (2018) 3248-3264. |
| [55] |
H.P. Wang, H.H. Zhang, X.Q. Hu, P.F. Xu, Y.C. Luo, Eur. J. Org. Chem. (2015) 3486-3494. |
| [56] |
X. Fu, L.L. Lin, Y. Xia, et al., Org. Biomol. Chem. 14 (2016) 5914-5917. DOI:10.1039/C6OB00948D |
| [57] |
R.K. Varshanya, P. Banerjee, Org. Biomol. Chem. 15 (2017) 5182-5190. DOI:10.1039/C7OB00941K |
 2020, Vol. 31
2020, Vol. 31