b University of Science and Technology of China, Hefei 230026, China;
c Department of Materials Science & Engineering & Department of Energy and Resources Engineering, College of Engineering, Peking University, Beijing 100871, China;
d School of Chemical and Environmental Engineering, Wuyi University, Jiangmen 529020, China
Due to the ever-growing energy crisis and environmental pollution, the demand for clean energy has never been as urgent as it is today [1-8]. The Gibbs free energy of fuel can be converted into electrical energy by electrochemical reaction, hence fuel cells can own a high efficiency which is not limited by the Carnot cycle laws [9, 10]. In addition, fuel cells are environmental friendly energy conversion devices from which few harmful gas would be emitted. From the perspective of saving energy and protecting the ecological environment, low temperature fuel cells are the most promising power generation technologies. On the anode of fuel cell, hydrogen or methanol is oxidized to generate electrons which move to the cathode through the external circuit. On the cathode, oxygen reduction reaction (ORR) occurs to generate water (O2 + 4H+ + 4e-→ 2H2O)in acidic electrolyte or hydroxyl ions (O2 + 2H2O + 4e-→ 4OH) in alkaline electrolyte [11]. The loss of efficiency in fuel cells mainly comes from the sluggish kinetics of ORR, which results in a large consumption of catalysts on the cathode [12]. Up to now, Pt nanoparticles supported on carbon blacks (Pt/C) is the most effective catalyst for ORR on the cathode in a fuel cell. However, the commercial Pt/C suffers from some intrinsic drawbacks: poor methanol tolerance and low cyclical stability caused by easy-to-aggregation [13, 14]. In addition, the high cost and limited reserve of Pt are also the bottlenecks of the large-scale application of fuel cells. Therefore, the fabrication of carbon-based catalysts with high-efficiency, durability, and anti-methanol ability for ORR is of great significance.
To design carbon-based catalysts competed with the commer-cial Pt/C for ORR, heteroatom doping, tuning the composition, fabricating hierarchical pore structures, and creating defects have been regarded as the practical and effective approaches [15-23]. Transition metal-nitrogen-carbon (M-N-C) species, in which M-Nx acts as the active site, have been recognized as the most promising ORR electrocatalysts for their high activity, good stability, and easy-to-preparation [24-27]. In recent years, single-atom doped carbon materials have drawn much attention as the prosperous electrocatalysts for ORR [28-30]. Fe, Co and Ni isolated single atoms have been doped into carbon materials by the ingenious and rational synthetic methods. With the development of advanced characterization techniques (in situ spectroscopy and atomic-resolution aberration-corrected scanning transmission electron microscopy) and theoretical calculations, the research on active sites for ORR is more comprehensive, which is conducive to capturing the nature of active sites [31, 32]. In view of the exciting advancements in this field, this review focuses on the recent progress of carbon-based catalysts for ORR. We highlight crucial geometric and electronic factors which affect the ORR electro-activity of carbon-based catalysts: conductivity, surface area, hierarchical porous structure, defect and doping effect. We emphasize that achieving the balance of the above crucial factors can lead to excellent ORR performances, including high activity, good stability, and sufficient resistance to methanol. Finally, we summarize the remaining challenges in this field and propose the specific direction of rational designation of carbon-based catalysts for ORR in the future.
2. ORR process 2.1. ORR processThe process of oxygen reduction is distinguished with the changes of the electrolyte and electrode materials. In alkaline solution, oxygen is transformed into OH by the 4-electron pathway (O2 + 2H2O + 4e-→ 4OH-). Otherwise, oxygen is transformed into HO2-, and HO2- is then transformed into OH-by the 2 + 2 electron pathway (O2 + H2O + 2e-→ HO2-+OH; HO2- +H2O + 2e-→ 3OH-). In acidic solution, oxygen molecule is transformed into H2O by the 4-electron pathway (O2 + 4H+ + 4e-→ 2H2O). Otherwise, oxygen is transformed into H2O2, and H2O2 is then transformed into H2O by the 2 + 2 electron pathway (O2 + 2H+ + 2e-→ H2O2; H2O2 + 2H+ +2e-→2H2O) [33-35].
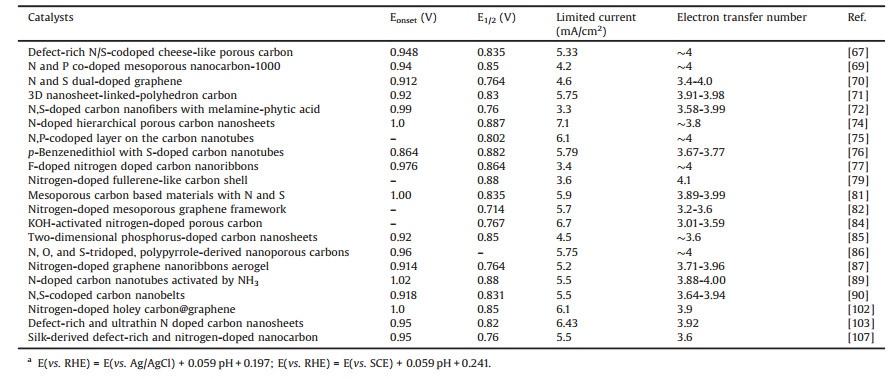
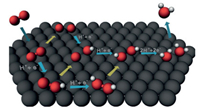
The thermodynamic equivalent potential of ORR is theoretically 1.229 V (O2 + 4H+ + 4e-→ 2H2O, E0 = 1.229 V vs. RHE). In fact, imperative overpotential is needed to drive ORR because the actual process of ORR on the electrode surface is complex. Research on the mechanism of ORR indicates that there are different pathways to achieve the reduction of oxygen and there are several intermedi-ates (O*, OH*, and OOH*) in the ORR process [36]. Under the actual conditions, oxygen firstly diffuses to the surface of the catalysts to form an activated O2* molecule, which is then converted into H2O by different pathways (Fig. 1) [37]. Under high oxygen concentra-tion, O2* molecule undergoes the association pathway. O2* firstly adsorbs onto the surface of the catalysts followed by the broken of O-O bond to form OH*. Under low oxygen concentration, O-O bond of O2* is firstly broken to form O* and O* then adsorbs onto the surface of the catalysts to form OH*, which is named as dissociation pathway. Another is the peroxo pathway, in which O2* molecule is coordinated with two protons to generate HOOH* followed by the cleavage of O-O bond to form H2O2 in acidic solution or HO2- in alkaline solution.
|
Download:
|
| Fig. 1. Illustration of different pathways for ORR. The red and white spheres represent oxygen and hydrogen atoms, respectively. The catalysts are represented by the black spheres. Copied with permission [37]. Copyright 2016, Wiley VCH. | |
2.2. Main factors for ORR
The ORR activities of carbon-based catalysts can be affected by many features from geometric and electronic structures. Specifi-cally, the performance of catalyst can be adjusted by the following parameters, such as conductivity, surface area, hierarchical porous structure, structural defects, and doping effect [38, 39]. Therefore, on the basis of ensuring good conductivity and high surface area of catalysts, reasonable regulation of the type and density of the active site is the key path to build catalysts that can be applied to practical energy storage and conversion systems. The excellent conductivity is the basic and essential factor of catalysts for the electrochemical process. For example, metal organic frameworks (MOFs) that possessing intrinsic metal-nitrogen coordination sites, high surface area, and abundant porous structures, have been seen as the popular precursors to prepare the ORR catalysts [40]. However, the poor conductivity of MOFs is the fatal drawback, which restricts the utilization of MOFs in the field of electro-catalysis [41, 42]. Therefore, to effectively enhance the conductivity of MOFs, pyrolysis-treatment for MOF and fabricating MOF/carbon hybrids have been regarded as effective ways to build efficient MOF-based ORR catalysts [43].
High surface area and hierarchical porous structure can promote the mass transfer process and maximize the exposure of active sites. In addition, the porous structure can facilitate the diffusion of relevant reactants and species. However, the over-robust porous structure will inevitably result in contact resistance and reduce the electron conductivity and mass transportation [44]. It is necessary to precisely fabricate the nanostructure to control the balance of porous structure and excellent conductivity. Recently, many studies report that defects can directly act as the active sites for ORR or provide the numerous anchor sites for the doping elements [45-47]. However, too much defects in the framework would decrease the conductivity of the catalytic material. For example, by changing the pyrolysis temperature, Lan and his coworkers search for the balance of 3D α-MnO2 microspheres between the defects and electron conductivity, which leads to the optimized ORR electrocatalysis [48]. Therefore, efficient control of the balance among the fatal factors can lead to the satisfied performance of ORR.
To achieve the ultra-high electrochemical ORR activity, the actual active sites in the carbon-based catalysts have been studied for a long time. In N-doped graphene and N-doped carbon nanotubes (CNTs), the C atoms adjoined N atoms are regarded as the active sites because the electronegative N atoms can tune the charge density of the carbon matrix [49]. In M-N-C species, M-Nx sites are recognized as the active centers for ORR, which is demonstrated by both the experiments and theoretical computa-tions [50-53]. Recently, defect-rich carbon nanomaterials have been demonstrated performing promising oxygen electroactivities because the electron distribution can be tuned and the p electron of carbon can be activated by the defects [54, 55]. It has been studied that defects can result in modulation of charge distribution and enhancement of charge density and then conduct as the active centers for ORR. The identification of the real active sites during the ORR process can deepen our understanding of the ORR catalytic process and promote the design of excellent ORR catalysts. Despite numerous efforts about searching active sites for ORR have been performed, the nature of active sites for ORR is still ambiguous and under debate, even for the N doped carbon, it is hard to determine which type of N is more effective in promoting ORR. Different precursors and synthetic methods inevitably cause the non-uniform and complex nanostructures of catalysts, which impede the identification of active catalytic sites. With the rapid development of aberration-corrected scanning transmission elec-tron microscopy (AC-STEM) and various in-situ spectroscopy, it is possible to study the active sites on the atomic level in the future [56].
Based on the previous progress on the carbon-based ORR catalysts, we can conclude the following guesses: the excellent conductivity is the basic factor for the electrochemical process of catalyst; high surface area and hierarchical porous structure can promote the mass transfer process and provide adequate contact sites; structural defects have been demon-strated as the high-efficient active site for the ORR process; last but not the least, the maximization of intrinsic activity of active sites by the doping effect is the most important and recognized mean of electronic structure optimization for the carbon-based ORR catalysts.
3. Carbon-based catalysts for ORR 3.1. Non-metal doped carbon materialsCarbon nanomaterials have been widely used as the ORR electrocatalysts for their reasonable balance of electron conductivity, surface area, porous structure, and low-cost [57-60]. However, the intrinsic carbon nanomaterials (graphene, CNTs, and carbon blacks) perform deficient ORR activity for the lack of active sites. Doping non-metal heteroatoms into 1D, 2D, and 3D carbon materials is effective to improve the electroactivity for oxygen reduction [61-67]. The enhanced ORR activity is achieved by modulating the electronic density and state of carbon matrix by the heteroatom whose electronegativity is different from that of carbon atom [68-70]. The single, dual, and triple heteroatoms (N, S, P and B) doped carbon materials (Table 1) perform Pt-like ORR performances, including high electroactivity, excellent durability, and good tolerance against methanol [71-92].
|
|
Table 1 Summary of non-metal doped carbon materials as ORR catalysts. The electroactivities of these catalysts are measured in 0.10 mol/L KOH solution.a |
3.1.1. Non-metal doped 1D carbon materials
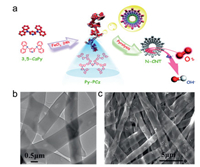
Non-metal doped 1D carbon materials such as CNTs, carbon nanofibers (CNFs), and carbon nanobelts (CNBs) have drawn much attention in the past decades. Vertically aligned N-doped CNTs were firstly prepared as high efficient metal-free ORR electro-catalysts in alkaline solution by Dai's group, which opened the new avenue to develop variety non-metal doped CNTs as ORR catalysts [93]. The theoretical calculations demonstrate that the N atoms adjacent to carbon cause the oxygen chemisorption change from the hard Pauling model (end-on adsorption) to the easy Yeager model (side-on adsorption), which can be attributed to the charge delocalization caused by the lower electronegativity of N atoms compared to C atoms. Most recently, Zhu et al. developed a new self-templated way to prepare polymeric nanotubes using 3, 5-difluoropyridine and carbazole as the precursors (Fig. 2a) [94]. The polymetric nanotubes were then converted into N-doped CNTs by pyrolyzing at 900 ℃ in N2 atmosphere and then activating in NH3 for another 15 min. The porous structure and rich N-doped active centers are essential for the excellent ORR activity of the prepared N-doped CNTs. The half-wave potential (E1/2) of the prepared N-doped CNTs is 0.88 V in 0.10 mol/L KOH, which is more positive than that of the commercial Pt/C (0.86 V). Our group fabricated N, S-codoped CNBs with blooming flowers (Figs. 2b and c) where the nanobelt structures were formed in the electrospinning procedure with phase separation and solvent evaporation [95]. In this work, both KCl and ZnCl2 play the important roles in the generation of micropores and nanobelt microstructures. In addition, the S dopant can increase the disordered carbon degree (defects) which is responsible for the ORR electroactivity. The resulting N, S-codoped CNBs performs boosting ORR activity in both alkaline and acidic solutions.
|
Download:
|
| Fig. 2. (a) Illustration of preparation of N-doped CNTs by the conjugated-nanoporous-polymer-driven, self-templated route. Copied with permission [94]. Copyright 2017, Royal Society of Chemistry. (b) TEM and (c) scanning electron microscope (SEM) images of N, S-codoped CNBs obtained by electrospinning technique. Reproduced with permission [95]. Copyright 2016, Royal Society of Chemistry. | |
3.1.2. Non-metal doped 2D carbon materials
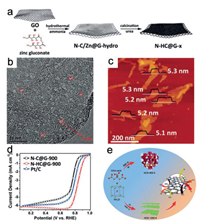
Among the 2D carbon materials, graphene is the most representative one, which can be synthesized by mechanical exfoliation, oxidation-reduction, and chemical vapor deposition [96, 97]. Graphene possesses the fascinating properties, such as large BET surface area (2630 m2/g in theory), excellent conductivi-ty, and good chemical stability, which are advantageous to the performances of oxygen reduction [98-101]. Recently, Sun and his coworkers synthesized an ultrathin N-doped holey carbon layer on a graphene sheet (N-HC@G-900) by a facile two-step method (Fig. 3a) [102]. The nanoholes (ca. 2 nm, Fig. 3b) derived from the evaporation of Zn are homogeneously dispersed on N-HC@G-900 whose average thickness is 5.2 nm (Fig. 3c). The rich edge sites on the nanoholes promote the selective generation of high-active pyridinic N for ORR. In addition, the unique sandwich-structure of N-HC@G-900 offers mechanical support, stabilizes the layered structure of holey carbon, and facilitates the charge transfer. The above structural merits lead to the excellent basic ORR activity of N-HC@G-900 with the onset potential (Eonset) and E1/2 to be 1.0 and 0.85 V from the linear sweep voltammetry (LSV), respectively (Fig. 3d). Jiang et al. put up a novel spontaneous gas-foaming strategy to fabricate ultrathin (2 nm) nanocarbon sheets (NCN-1000-5) by annealing a mixture of NH4Cl and citric acid at 1000 ℃ (Fig. 3e) [103]. They demonstrate that the annealing temperature is important to control the thickness and porosity of the carbon nanosheets. The thickness of carbon nanosheets slowly decreases with the annealing temperature increasing (from 800 ℃ to 1000 ℃). Confirmed by both experimental and calculation data, the graphitic N doping and substantial edge defects on the nanoporous structure give rise to the commercial Pt-like activity and intensive stability of NCN-1000-5 for ORR.
|
Download:
|
| Fig. 3. (a) Schematics illustration for the formation of N-HC@G-900. (b) HRTEM image of N-HC@G-900 (red circles: the formed nanoholes). (c) Atomic force microscope (AFM) image of N-HC@G-900. (d) LSV curves in 0.10 mol/L KOH. Reproduced with permission [102]. Copyright 2018, Wiley VCH. (e) The schematic procedure for synthesizing of NCN-1000-5. Copied with permission [103]. Copyright 2019, Royal Society of Chemistry. | |
3.1.3. Non-metal doped 3D carbon materials
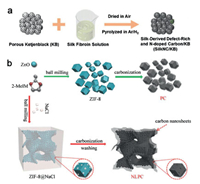
The unique 3D porous carbon catalysts provide high surface area, hierarchical porous structures, rich defects, and good electrical conductivity, which can boost the oxygen reduction [104-106]. For example, Zhang's group prepared N-doped defect-rich metal-free catalysts by a mild method with biomass material (silk) as C and N precursors (Fig. 4a) [107]. Biomass derived from plants (seaweed, soybeans, gingko leaves, coconut shells, and fresh grass) or animals (human hairs, poultry feathers, shrimp shells, egg, and cocoon silk) are ideal precursors to prepare active carbon materials for ORR because biomass materials are abundant, low-cost, and renewable resources [108-118]. The synthesized catalyst features pyridinic and graphitic N-dominated nitrogen species, high surface area, porous structure, and good conductivity, which are beneficial to the exposure of active sites and electron and mass transfer for ORR. The biomass-derived architecture exhibits not only good activity (Eonset of 0.95 V) and long-term stability for ORR but also good performance for Zn-air batteries.
|
Download:
|
| Fig. 4. (a) Illustration of the fabrication of SilkNC/KB. Copied with permission [107]. Copyright 2019, American Chemical Society. (b) Schematic presentation for synthesis of NLPC. Copied with permission [75]. Copyright 2018, Wiley VCH. | |
Template method is effective to prepare 3D porous carbon catalysts, for example, silica and metal oxides can be used as the typical hard templates while surfactants such as Pluronic P123 and Pluronic F127 can be used as the typical soft templates [119-124]. The abundant porous structures created by the elimination of the template can maximize the exposure of active sties and promote the mass transport during ORR [125, 126]. Wang's group prepared the defect-rich 3D nanosheet-linked-polyhedron carbon (NLPC) with NaCl was used as the salt reactor and template (Fig. 4b) [75]. The confinement effect of NaCl template prevents the collapse and loss of abundant carbon structures compared with the direct pyrolysis of MOFs, which can reserve more active sites for ORR electrocatalysis. The unique 3D linking of carbon nanosheets and carbon polyhedrons derived from the NaCl template can facilitate the mass transport for ORR and increase the electron conductivity. In addition, the elimination of NaCl template results in the ample edge structures of carbon matrix, which is helpful to promote the ORR activity. The prepared 3D NLPC possesses the commercial Pt-like electroactivity for oxygen reduction with low H2O2 yield (below 4.5%) and high electron transfer number (3.91-3.98).
3.2. Transition metal doped carbon materialsAlthough metal-free catalysts have been widely explored for ORR due to their low-cost and long-term stability, their ORR activity especially in acidic solution cannot meet the requirement of substituting for the commercial Pt/C. Metal-nitrogen-carbon (M-N-C) and metal carbides (M3C) based carbon hybrids are the most attractive electrocatalysts for ORR especially in acidic electrolyte (Table 2), where M represents for transition metal (Fe and Co) [127-148]. Since Jasinski firstly discovered that cobalt phthalocyanine was effective for the reduction of oxygen under alkaline condition in 1964, M-N4 macrocycles (porphyrins, pathalocyanines, and tetraazaannulenes) were then developed as ORR electrocatalysts despite of their high cost, poor thermal stabilities, and poor conductivities [149]. Series of M-N-C materials were subsequently prepared by pyrolyzing various transition metal, nitrogen, and carbon precursors in inert atmosphere. The O¼O bond is prone to be broken by the central metal, which greatly promotes the reduction of oxygen on the surface of M-N-C catalysts. In general, many researchers confirm that M-Nx sites in M-N-C catalysts are active centers for ORR [150-152]. M-N4 units, as the basic structural blocks in metal macrocycles, are demon-strated as the active sites for ORR by Bouwkamp-Wijnoltz using in-situ Mossbauer spectroscopy [49]. However, many researchers argue that transition central metal plays the role in the promotion of graphitization and formation of different type of nitrogen (pyridinic, pyrrolic, and graphitic N), instead of acting as the active sites for ORR. First-principles calculations prove that pyridinic and graphitic N act as the main active sites in N-doped carbon materials [153]. Graphitic N determines the limited charge density and pyridinic N is favorable to enhance the onset potential of oxygen reduction [154]. Despite many efforts have been made, the intrinsic nature of active sites for ORR in M-N-C species is still under debate.
|
|
Table 2 Summary of M-N-C/M3C species as ORR catalysts.a |
3.2.1. M-N-C/M3C doped carbon catalysts
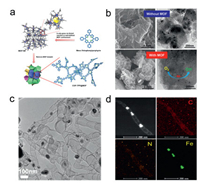
Direct pyrolysis of the mixture of metal salts, nitrogen, and carbon precursors is the facile method to prepare M-N-C/M3C catalysts [155-163]. However, the physical mixing of these precursors and subsequent heat-treatment of the mixtures may cause the inhomogeneous microstructures, which lead to the nonuniform distribution of metal-based active sites. As the ordered coordination of metal ions and organic ligands, MOFs are the promising precursors to prepare porous M-N-C/M3C catalysts for ORR [164-170]. The intrinsic coordination of metal with nitrogen and abundant porous structures of MOFs are accessible to provide rich and homogeneous M-Nx sites for oxygen reduction [134, 171-173]. Most recently, Dai's group designed rod-like Co@NC catalysts by pyrolyzing a novel 3D enantiotopic chiral MOFs derived from the coordination interaction of Co2+, MIDPPA (4, 4'-di(4-pyridine)-4"-imidazoletriphenylamine), and 1, 2, 4-btc (1, 2, 4-benzenetricarboxylic acid) [174]. The helices structures of the crystals give rise to the rod-like 3D MOF structures and the rod-like structures keep well in annealing with the resistance of the stable ligand (MIDPPA). The catalyst performs commercial Pt-like ORR activity with the E1/2 of 0.82 V. Xiang et al. grow covalent organic polymers (COPs) within the well-defined space of MOF-180 which possesses high surface area and high porosity, followed by heat treatment to obtain COP@MOF-900 (Fig. 5a) [175]. Compared with the sample synthesized without the confinement of MOF-180, the COP@MOF-900 has smaller and more uniform dispersed Fe-N active sites for ORR (Fig. 5b), which is demonstrated by X-ray absorption fine structure measurements and first-principles calculations. The confinement effect of ordered MOF-180 contributes to the controllable morphology of COP@MOF-900 and the controllable size of the metal nanoparticles.
|
Download:
|
| Fig. 5. (a) Synthetic process of COP@MOF. (b) SEM and TEM images of the catalysts prepared without and with MOF. Reproduced with permission [175]. Copyright 2017, American Chemical Society. (c) TEM image of Fe3C nanoparticles/bamboo-like CNTs hybrids. (d) HAADF-STEM and the corresponding elemental mapping images of the sample. Reproduced with permission [186]. Copyright 2015, American Chemical Society. | |
The generation of Fe-N-C active sites may be accompanied by the formation of Fe3C nanoparticles during the pyrolysis. Many researchers propose that the excellent ORR electroactivity can be achieved by the synergistic effect between Fe-Nx and Fe3C crystals [176-181]. Wan's group demonstrates that Fe3C nanoparticles boost the electroactivity of Fe-Nx by the well-designed experi-ments and DFT calculation [158]. Yu's group also proves that the excellent ORR activity can be supported by the synergetic effect of the two coexistence active species (Fe-Nx and Fe3C) [15]. However, many other researchers find that Fe3C nanoparticles still perform the promising ORR activity without Fe-Nx [182-185]. Hu et al. synthesized Fe3C nanoparticles encapsulated by graphitic layers with little nitrogen on the surface of catalysts [130]. By ball-milling and acid-leaching operations, the encased Fe3C nanoparticles are removed from the catalysts and the remaining sample shows poor activity for ORR. It can be concluded that the Fe3C crystals play the key role in catalyzing oxygen reduction by activating the surrounding graphitic layers. Our group developed a new soft-template-induced method to prepare Fe3C nanoparticles/bamboo-like CNTs hybrids (Figs. 5c and d) by pyrolyzing the mixture of Fe (NO3)3, melamine, and Pluronic P123 [186]. P123 acts as the 1D soft-template which assists the formation of bamboo-like CNTs by connecting rolled carbon units. The wrapped Fe3C nanoparticles can promote the work function of the graphitic layers and the bamboo-like feature can provide more edges of graphitic carbon, both of which are beneficial for improving the activity of ORR. The prepared hybrids perform not only excellent ORR electroactivity in alkaline solution but also good ORR activity in acidic solution.
3.2.2. Single atom catalystsAlthough M-N-C/M3C doped carbon catalysts perform good activity for ORR, however, it has been demonstrated that the inner metal atoms in the nanoparticles cannot be utilized effectively, and the electroactivity and durability can be promoted through decreasing the size of active metal nanoparticles [174, 187, 188]. In addition, modifying the coordination chemical environment of the central metal atoms is more available for isolated dispersed metal atoms. Therefore, single atom catalysts (SACs), as an emerging catalysts with the metal atoms are isolated dispersed on the substrate, have aroused extensive interests due to the advantages of unique electronic effect, homogeneous distribution of active sites, and maximum utilization efficiency of metal [189-193].
MOFs have been utilized as appropriate precursors to fabricate SACs for the unique ability to generate homogeneous M-Nx active centers and porous structures via thermal activation. Most recently, Wu's group designed a novel surfactant-assisted ZIF-8 method to yield atomically dispersed Co-N4 nanocarbon (Co-N-C@F127) as ORR catalyst (Fig. 6a) [194]. The intimate interaction between ZIF-8 and F127 leads to the unique confine-ment effect, which plays a key role in impeding the aggregation of single Co atoms and keeping the intrinsic porous structures. The atomically dispersed Co atoms can be proved by aberration-corrected HAADF-STEM image (Fig. 6b), X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectroscopy. It has been indicated that Co-N-C@F127 possesses the relatively larger density of active sites compared with the samples prepared without F127. Co-N-C@F127 performs excellent ORR activity in 0.50 mol/L H2SO4 with the E1/2 is as positive as 0.84 V (Fig. 6c). Miao and his coworkers prepared single Fe atoms dispersed on N-doped porous carbon (SA-Fe-N) by polymerization of Fe3+ and sodium alginate to form metal-organic polymer coordinative hydrogel [195]. Fe3+ can be effectively confined and stabilized via chelation reaction between Fe3+ and sodium alginate, which leads to the isolated Fe atoms on the carbon matrix. The SA-Fe-N performs outstanding ORR electro-activity in both 0.10 mol/L KOH and 0.50 mol/L H2SO4 with the E1/2 of 0.910 and 0.812 V, respectively.

|
Download:
|
| Fig. 6. (a) The strategy to in situ confinement annealing to prepare Co-N-C@F127. (b) Magnified aberration-corrected HAADF-STEM image of Co-N-C@F127. (c) ORR polarization curves of Co-N-C@F127 and commercial Pt/C in 0.50 mol/L H2SO4 at 900 rpm. Reproduced with permission [194]. Copyright 2019, Royal Society of Chemistry. | |

|
Download:
|
| Fig. 7. (a) Illustration of the formation of M-N-C (M = Co and Fe) single-atom electrocatalysts. (b) TEM and HAADF-STEM (inset) images of Fe-N-C-900. (c) Magnified aberration-corrected HAADF-STEM image of Fe-N-C-900. (d) ORR polarization curves of the catalysts in 0.10 mol/L KOH at 1600 rpm. (e) Free energy diagram of ORR on a FeN2 and on CoN2 sites. Reproduced with permission [198]. Copyright 2018, Wiley VCH. | |
SACs have also been prepared with diverse templates (SiO2 spheres, FeOOH nanorods, MgO, etc.) [196-199]. Zhu and his coworkers developed single-atom Fe/Co-N-C catalysts with hierarchical pores by a bimodel template (SiO2 and ZnCl2) approach (Fig. 7a) [198]. TEM and HAADF-STEM images (Fig. 7b) of Fe-N-C-900 evidently illustrate the well-ordered spherical porous nanostructures arised from the SiO2 nanospheres stacking with precursors, on which there is no obvious metal nanoparticles or nanoclusters. Further, atomically dispersed Fe atoms can be observed in the magnified aberration-corrected HAADF-STEM image (Fig. 7c). The chemical coordination information of central Fe/Co atoms are confirmed to be Fe(Co)N2 by HAADF-STEM image, XANES, and EXAFS. Polarization curves (Fig. 7d) demonstrate that Fe-N-C displays more positive E1/2 (0.927 V) than that of Co-N-C (0.878 V) because FeN2 active structures in Fe-N-C-900 facilitate the rate-limiting release of OH* and enhance ORR process compared with CoN2 moieties in Co-N-C-900, which can be concluded from DFT calculations (Fig. 7e).
3.2.3. Transition metal oxides/chalcogenides/phosphides carbon hybridsAlthough transition metal oxides, chalcogenides, and phos-phides nanoparticles perform prospective electroactivity for ORR, the wide application of them is hindered by the low electrical conductivity, low surface area, and easy-to-aggregation. Growing transition metal oxides, chalcogenides and phosphides nanoparticles on the carbon substrate can overcome these challenges and facilitate the ORR activity to a great extent [200-225]. More recently, Guan et al. synthesized hollow nano-spheres of Co3O4 anchored within N-doped porous carbon nanoarrays (NC-Co3O4) as ORR and OER bi-functional electro-catalysts [215]. The as-prepared Co-MOF nanoarrays on carbon cloth are transformed into porous carbon with solid Co nano-particles by heating in Ar/H2 flow, and subsequently transformed into porous carbon with hollow Co3O4 nanospheres by annealing in air. Such a binder-free catalyst can be directly used as electrode in the test cell and NC-Co3O4 is effective for oxygen reduction in alkaline solution.
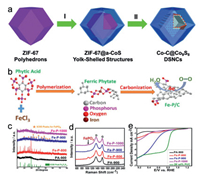
Co-C@Co9S8 double-shelled nanocages (DSNCs) (Fig. 8a) were designed by Lou's group through the reflux process to grow CoS shell outside ZIF-67 polyhedrons, followed by heat treatment to transform CoS and ZIF-67 to Co9S8 shells and carbon nanocages with Co nanoparticles, respectively [203]. It has been proved that the inner carbon nanocages loaded with Co nanoparticles mainly catalyze the oxygen reduction, while the outer Co9S8 shells provide fast mass transfer to the inner shells and protect the inner shells from aggregation and etching. Co-C@Co9S8 DSNCs display the good ORR activity and stability in 0.10 mol/L KOH, which can be attributed to the profitable double-shelled structure.
Yu's group developed Fe-P bonded carbon catalysts with phytic acid and FeCl3 (Fig. 8b) [201]. The six negatively charged phosphate groups in phytic acid strongly coordinate with Fe3+ to form ferric phytate, followed by annealing at high temperature in N2 atmosphere. With the increase of the annealing temperature (from 800 ℃ to 1000 ℃), the less stable Fe2P4O12 decomposes and FePO4 appears gradually, which are confirmed by XRD (Fig. 8c) and Raman characterizations (Fig. 8d). The ORR activity of Fe-P bonded carbon is higher than that of the P-doped carbon prepared without Fe, which demonstrates that Fe-P bond makes the great contribu-tion to the ORR activity. Fe-P-900 performs the good ORR electroactivity comparable to the commercial Pt/C in both alkaline and acidic solutions (Fig. 8e), which confirms that Fe-P species are new class of active catalysts for ORR.
|
Download:
|
| Fig. 8. (a) Preparation of Co-C@Co9S8 double-shelled nanocages. Copied with permission [203]. Copyright 2016, Royal Society of Chemistry. (b) Illustration of synthesis of Fe-P bonded carbon catalysts with phytic acid and FeCl3. (c) XRD patterns and (d) Raman spectra of the prepared Fe-P bonded carbon samples. (e) Polarization curves of the prepared samples in O2-saturated 0.10 mol/L HClO4 solution. Reproduced with permission [201]. Copyright 2015, American Chemical Society. | |
4. Conclusions and outlook
In this review, we summarize recent progress on fabricating carbon-based catalysts for ORR which is critical for the construc-tion of renewable energy conversion and storage technologies. Heteroatom-doped carbon materials, M-N-C/M3C doped carbon species, single atom catalysts and other carbon hybrids have been extensively studied based on the earth-abundant elements. M-N-C/M3C doped carbon species have been considered as the most promising candidates for the commercial Pt/C in both alkaline and acidic solutions in the recent decades. Remarkably, maximizing the utilization of metal atoms and the density of active sites can lead to the formation of the SACs which possess the great potential to discover the nature of the ORR active sites and promote the wide application of ORR-based energy storage and conversion devices.
Although many promising materials have been prepared for ORR via variety rational and controllable strategies (geometric and electronic structures), the existed carbon-based catalysts still cannot substitute the standard Pt/C in the commercial production of fuel cells. Our personal prospects of the potential carbon-based catalysts for ORR are put forward as follows. (i) The accurate identifications of mechanism and active sites are the key issues for ORR, which can guide the rational and effective design of the ORR catalysts. Therefore, the theoretical calculation models should be closer to the actual experiment conditions, and advanced synthesis procedures should be used to produce ideal nanocatalysts. (ii) From transition metal nanoparticles to SACs, researchers studied the ORR activity on the smaller scale. Studies at the atomic level are more beneficial for revealing the active sites for ORR with the advanced characterization techniques. (iii) Synergetic effect among conductivity, surface area, porous structure, and defects can promote the ORR performance, and rational engineering the balance of these geometric and electronic factors can lead to a better ORR performance of the catalysts, even extends to the wider catalysis systems.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21675147 and 21802003), the Jilin Provincial Science and Technology Development Program (Nos. 20190201242JC and 20180520142JH) and the China Postdoctoral Science Foundation (No. 2018M631239).
| [1] |
A. Kulkarni, S. Siahrostami, A. Patel, J.K. Norskov, Chem. Rev. 118 (2018) 2302-2312. DOI:10.1021/acs.chemrev.7b00488 |
| [2] |
M.L. Pegis, C.F. Wise, D.J. Martin, J.M. Mayer, Chem. Rev. 118 (2018) 2340-2391. DOI:10.1021/acs.chemrev.7b00542 |
| [3] |
X. Yan, Y. Jia, X. Yao, Chem. Soc. Rev. 47 (2018) 7628-7658. DOI:10.1039/C7CS00690J |
| [4] |
A.A. Gewirth, J.A. Varnell, A.M. DiAscro, Chem. Rev. 118 (2018) 2313-2339. DOI:10.1021/acs.chemrev.7b00335 |
| [5] |
W. Yang, Y. Zhang, C. Liu, J. Jia, J. Power Sources 274 (2015) 595-603. DOI:10.1016/j.jpowsour.2014.10.067 |
| [6] |
M. Chen, L. Wang, H. Yang, et al., J. Power Sources 375 (2018) 277-290. DOI:10.1016/j.jpowsour.2017.08.062 |
| [7] |
L. Chen, W. Yang, X. Liu, J. Jia, Int. J. Hydrogen Energy 42 (2017) 12246-12253. DOI:10.1016/j.ijhydene.2017.03.054 |
| [8] |
M. Borghei, J. Lehtonen, L. Liu, O.J. Rojas, Adv. Mater. 30 (2018) 1703691.
|
| [9] |
S. Guo, S. Zhang, S. Sun, Angew. Chem. Int. Ed. 52 (2013) 8526-8544. DOI:10.1002/anie.201207186 |
| [10] |
A.A. Gewirth, M.S. Thorum, Inorg. Chem. 49 (2010) 3557-3566. DOI:10.1021/ic9022486 |
| [11] |
M. Shao, Q. Chang, J.P. Dodelet, R. Chenitz, Chem. Rev. 116 (2016) 3594-3657. DOI:10.1021/acs.chemrev.5b00462 |
| [12] |
T. Sun, B. Tian, J. Lu, C. Su, J. Mater. Chem. A:Mater. Energy Sustain. 5 (2017) 18933-18950. DOI:10.1039/C7TA04915C |
| [13] |
B. Wang, X. Cui, J. Huang, R. Cao, Q. Zhang, Chin. Chem. Lett. 29 (2018) 1757-754. DOI:10.1016/j.cclet.2018.11.021 |
| [14] |
W. Gu, L. Hu, J. Li, E. Wang, Electroanalysis 30 (2018) 1217-1228. DOI:10.1002/elan.201700780 |
| [15] |
Z.Y. Wu, X.X. Xu, B.C. Hu, et al., Angew. Chem. Int. Ed. 54 (2015) 8179-8183. DOI:10.1002/anie.201502173 |
| [16] |
H.W. Liang, W. Wei, Z.S. Wu, X. Feng, K. Mullen, J. Am. Chem. Soc. 135 (2013) 16002-16005. DOI:10.1021/ja407552k |
| [17] |
S. Zhao, L. Yan, H. Luo, W. Mustain, H. Xu, Nano Energy 47 (2018) 172-198. DOI:10.1016/j.nanoen.2018.02.015 |
| [18] |
Y. Xue, S. Sun, Q. Wang, Z. Dong, Z. Liu, J. Mater. Chem. A:Mater. Energy Sustain. 6 (2018) 10595-10626. DOI:10.1039/C7TA10569J |
| [19] |
Q. Ren, H. Wang, X.F. Lu, Y.X. Tong, G.R. Li, Adv. Sci. 5 (2018) 1700515.
|
| [20] |
Y. Lu, Y. Ma, T. Zhang, et al., J. Am. Chem. Soc. 140 (2018) 11538-11550. DOI:10.1021/jacs.8b06414 |
| [21] |
C. Zhu, H. Li, S. Fu, D. Du, Y. Lin, Chem. Soc. Rev. 45 (2016) 517-531. DOI:10.1039/C5CS00670H |
| [22] |
D. Yan, Y. Li, J. Huo, et al., Adv. Mater. 29 (2017) 1606459.
|
| [23] |
C. Tang, Q. Zhang, Adv. Mater. 29 (2017) 1604103.
|
| [24] |
C. Zhang, J. Liu, Y. Ye, et al., ACS Appl. Mater. Interfaces 10 (2018) 2423-2429. DOI:10.1021/acsami.7b14443 |
| [25] |
D. Higgins, P. Zamani, A. Yu, Z. Chen, Energy Environ. Sci. 9 (2016) 357-390. DOI:10.1039/C5EE02474A |
| [26] |
S. Fu, C. Zhu, D. Su, et al., Small 14 (2018) 1703118.
|
| [27] |
V. Armel, S. Hindocha, F. Salles, et al., J. Am. Chem. Soc. 139 (2017) 453-464. DOI:10.1021/jacs.6b11248 |
| [28] |
Z. Zhang, J. Sun, F. Wang, L. Dai, Angew. Chem. Int. Ed. 57 (2018) 9038-9043. DOI:10.1002/anie.201804958 |
| [29] |
H. Zhang, W. Zhou, T. Chen, et al., Energy Environ. Sci. 11 (2018) 1980-1984. DOI:10.1039/C8EE00901E |
| [30] |
Z.K. Yang, C.Z. Yuan, A.W. Xu, Nanoscale 10 (2018) 16145-16152. DOI:10.1039/C8NR04372H |
| [31] |
Y. Wang, H. Yuan, Y. Li, Z. Chen, Nanoscale 7 (2015) 11633-11641. DOI:10.1039/C5NR00302D |
| [32] |
B. Lu, T.J. Smart, D. Qin, et al., Chem. Mater. 29 (2017) 5617-5628. DOI:10.1021/acs.chemmater.7b01265 |
| [33] |
Y. Zheng, Y. Jiao, M. Jaroniec, Y. Jin, S.Z. Qiao, Small 8 (2012) 3550-3566. DOI:10.1002/smll.201200861 |
| [34] |
J. Zhang, K. Sasaki, E. Sutter, R.R. Adzic, Science 315 (2007) 220-222. DOI:10.1126/science.1134569 |
| [35] |
D. Yu, E. Nagelli, F. Du, L. Dai, J. Phys. Chem. Lett. 1 (2010) 2165-2173. DOI:10.1021/jz100533t |
| [36] |
J.K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. Phys. Chem. B 108 (2004) 17886-17892. DOI:10.1021/jp047349j |
| [37] |
W. Xia, A. Mahmood, Z. Liang, R. Zou, S. Guo, Angew. Chem. Int. Ed. 55 (2016) 2650-2676. DOI:10.1002/anie.201504830 |
| [38] |
J. Ortiz-Medina, Z. Wang, R. Cruz-Silva, et al., Adv. Mater. 31 (2019) 1805717.
|
| [39] |
L. Wang, M. Gennari, Cantu Reinhard F.G., et al., J. Am. Chem. Soc. 141 (2019) 8244-8253. |
| [40] |
H. Zhang, H. Osgood, X. Xie, Y. Shao, G. Wu, Nano Energy 31 (2017) 331-350. DOI:10.1016/j.nanoen.2016.11.033 |
| [41] |
P.Q. Liao, J.Q. Shen, J.P. Zhang, Coord. Chem. Rev. 373 (2018) 22-48. DOI:10.1016/j.ccr.2017.09.001 |
| [42] |
L. Sun, M.G. Campbell, M. Dinca, Angew. Chem. Int. Ed. 55 (2016) 3566-3579. DOI:10.1002/anie.201506219 |
| [43] |
Y. Hou, T. Huang, Z. Wen, S. Mao, S. Cui, J. Chen, Adv. Energy Mater. 4 (2014) 1400337. https://www.zhangqiaokeyan.com/academic-journal-foreign_other_thesis/020413129768.html
|
| [44] |
M. Shen, C. Wei, K. Ai, L. Lu, Nano Res. 10 (2017) 1449-1470. DOI:10.1007/s12274-016-1400-7 |
| [45] |
H. Zhao, C. Sun, Z. Jin, et al., J. Mater. Chem. A:Mater. Energy Sustain. 3 (2015) 11736-11739. DOI:10.1039/C5TA02229K |
| [46] |
S.T. Senthilkumar, S.O. Park, J. Kim, et al., J. Mater. Chem. A:Mater. Energy Sustain. 5 (2017) 14174-14181. DOI:10.1039/C7TA03298F |
| [47] |
Z. Lu, J. Wang, S. Huang, et al., Nano Energy 42 (2017) 334-340. |
| [48] |
B. Lan, X. Zheng, G. Cheng, et al., Electrochim. Acta 283 (2018) 459-466. DOI:10.1016/j.electacta.2018.06.195 |
| [49] |
D. Guo, R. Shibuya, C. Akiba, et al., Science 351 (2016) 361-365. DOI:10.1126/science.aad0832 |
| [50] |
Y.J. Wang, D.P. Wilkinson, J. Zhang, Chem. Rev. 111 (2011) 7625-7651. DOI:10.1021/cr100060r |
| [51] |
A. Sarapuu, E. Kibena-Põldsepp, M. Borghei, K. Tammeveski, J. Mater. Chem. A:Mater. Energy Sustain. 6 (2018) 776-804. DOI:10.1039/C7TA08690C |
| [52] |
S. Liu, Z. Yang, M. Li, et al., Int. J. Hydrogen Energy 43 (2018) 5163-5174. DOI:10.1016/j.ijhydene.2018.01.151 |
| [53] |
K. Ai, Z. Li, X. Cui, J. Power Sources 368 (2017) 46-56. DOI:10.1016/j.jpowsour.2017.09.067 |
| [54] |
C. Tang, B. Wang, H.F. Wang, Q. Zhang, Adv. Mater. 29 (2017) 1703185.
|
| [55] |
Y. Jia, L. Zhang, A. Du, et al., Adv. Mater. 28 (2016) 9532-9538. DOI:10.1002/adma.201602912 |
| [56] |
H.T. Chung, D.A. Cullen, D. Higgins, et al., Science 357 (2017) 479-484. DOI:10.1126/science.aan2255 |
| [57] |
C. Hu, L. Dai, Angew. Chem. Int. Ed. 55 (2016) 11736-11758. DOI:10.1002/anie.201509982 |
| [58] |
J. Xu, L. Shi, J. Wang, et al., Carbon 138 (2018) 348-356. DOI:10.1016/j.carbon.2018.07.013 |
| [59] |
D. Liu, L. Dai, X. Lin, et al., Adv. Mater. 31 (2019) 1804863.
|
| [60] |
L. Yang, J. Shui, L. Du, et al., Adv. Mater. 31 (2019) 1804799.
|
| [61] |
Q. Li, R. Cao, J. Cho, G. Wu, Adv. Energy Mater. 4 (2014) 1301415. https://www.readcube.com/articles/10.1002/aenm.201301415
|
| [62] |
P. Matter, L. Zhang, U. Ozkan, J. Catal. 239 (2006) 83-96. DOI:10.1016/j.jcat.2006.01.022 |
| [63] |
J. Zhang, L. Qu, G. Shi, et al., Angew. Chem. Int. Ed. 55 (2016) 2230-2234. DOI:10.1002/anie.201510495 |
| [64] |
H. Yu, L. Shang, T. Bian, et al., Adv. Mater. 28 (2016) 5080-5086. DOI:10.1002/adma.201600398 |
| [65] |
S.S. Shinde, C.H. Lee, A. Sami, et al., ACS Nano 11 (2017) 347-357. DOI:10.1021/acsnano.6b05914 |
| [66] |
K. Qu, Y. Zheng, S. Dai, S.Z. Qiao, Nano Energy 19 (2016) 373-381. DOI:10.1016/j.nanoen.2015.11.027 |
| [67] |
Q. Liu, Y. Wang, L. Dai, J. Yao, Adv. Mater. 28 (2016) 3000-3006. DOI:10.1002/adma.201506112 |
| [68] |
J.J. Xu, C.H. Xiao, S.J. Ding, Chin. Chem. Lett. 28 (2017) 748-754. DOI:10.1016/j.cclet.2016.12.006 |
| [69] |
Y. Dong, M. Liu, Y. Liu, S. Wang, J. Li, J. Mater. Chem. A:Mater. Energy Sustain. 3 (2015) 19969-19973. DOI:10.1039/C5TA04624F |
| [70] |
M. Zhang, L. Dai, Nano Energy 1 (2012) 514-517. DOI:10.1016/j.nanoen.2012.02.008 |
| [71] |
J. Zhu, W. Li, S. Li, et al., Small 14 (2018) 1800563.
|
| [72] |
L. Zhao, X.L. Sui, J.Z. Li, et al., Appl. Catal. B 231 (2018) 224-233. DOI:10.1016/j.apcatb.2018.03.020 |
| [73] |
J. Zhang, Z. Zhao, Z. Xia, L. Dai, Nat. Nanotech. 10 (2015) 444-452. DOI:10.1038/nnano.2015.48 |
| [74] |
J.M. You, M.S. Ahmed, H.S. Han, et al., J. Power Sources 275 (2015) 73-79. DOI:10.1016/j.jpowsour.2014.10.174 |
| [75] |
Y. Wang, L. Tao, Z. Xiao, et al., Adv. Funct. Mater. 28 (2018) 1705356. https://onlinelibrary.wiley.com/doi/abs/10.1002/adfm.201705356
|
| [76] |
A. Mulyadi, Z. Zhang, M. Dutzer, W. Liu, Y. Deng, Nano Energy 32 (2017) 336-346. DOI:10.1016/j.nanoen.2016.12.057 |
| [77] |
Z. Ma, S. Dou, A. Shen, et al., Angew. Chem. Int. Ed. 54 (2015) 1888-1892. DOI:10.1002/anie.201410258 |
| [78] |
L. Liu, G. Zeng, J. Chen, et al., Nano Energy 49 (2018) 393-402. DOI:10.1016/j.nanoen.2018.04.061 |
| [79] |
Z. Li, W. Zhao, C. Yin, et al., ACS Appl. Mater. Interfaces 9 (2017) 44519-44528. DOI:10.1021/acsami.7b14815 |
| [80] |
R. Chen, J. Yan, Y. Liu, J. Li, J. Phys. Chem. C 119 (2015) 8032-8037. DOI:10.1021/acs.jpcc.5b00306 |
| [81] |
J. He, Y. He, Y. Fan, et al., Carbon 124 (2017) 630-636. DOI:10.1016/j.carbon.2017.08.081 |
| [82] |
Y. Han, D. Tang, Y. Yang, et al., Nanoscale 7 (2015) 5955-5962. DOI:10.1039/C4NR07116F |
| [83] |
S. Gao, X. Wei, H. Fan, et al., Nano Energy 13 (2015) 518-526. DOI:10.1016/j.nanoen.2015.02.031 |
| [84] |
M. Favaro, L. Ferrighi, G. Fazio, et al., ACS Catal. 5 (2014) 129-144. |
| [85] |
Z. Yan, L. Gao, C. Dai, et al., Int. J. Hydrogen Energy 43 (2018) 3705-3715. DOI:10.1016/j.ijhydene.2018.01.013 |
| [86] |
H.F. Wang, C. Tang, Q. Zhang, Catal. Today 301 (2018) 25-31. DOI:10.1016/j.cattod.2017.02.012 |
| [87] |
A. Tiwari, V. Singh, D. Mandal, T.C. Nagaiah, J. Mater. Chem. A:Mater. Energy Sustain. 5 (2017) 20014-20023. DOI:10.1039/C7TA05503J |
| [88] |
G. Lin, R. Ma, Y. Zhou, et al., Electrochim. Acta 261 (2018) 49-57. DOI:10.1016/j.electacta.2017.12.107 |
| [89] |
W. Lei, Y.P. Deng, G. Li, et al., ACS Catal. 8 (2018) 2464-2472. DOI:10.1021/acscatal.7b02739 |
| [90] |
Y. Meng, D. Voiry, A. Goswami, et al., J. Am. Chem. Soc. 136 (2014) 13554-13557. DOI:10.1021/ja507463w |
| [91] |
L. Chen, R. Du, J. Zhu, et al., Small 11 (2015) 1423-1429. DOI:10.1002/smll.201402472 |
| [92] |
K. Gao, B. Wang, L. Tao, et al., Adv. Mater. 31 (2019) 1805121.
|
| [93] |
K. Gong, F. Du, Z. Xia, M. Durstock, L. Dai, Science 323 (2009) 760-764. DOI:10.1126/science.1168049 |
| [94] |
X. Zhu, Y. Zhu, C. Tian, et al., J. Mater. Chem. A:Mater. Energy Sustain. 5 (2017) 4507-4512. DOI:10.1039/C6TA09604B |
| [95] |
W. Yang, L. Chen, X. Liu, et al., J. Mater. Chem. A:Mater. Energy Sustain. 4 (2016) 5834-5838. DOI:10.1039/C6TA01263A |
| [96] |
Y. Wang, D. Liu, Z. Liu, et al., Chem. Commun. (Camb.) 52 (2016) 12614-12617. DOI:10.1039/C6CC06608A |
| [97] |
C. Tan, X. Cao, X.J. Wu, et al., Chem. Rev. 117 (2017) 6225-6331. DOI:10.1021/acs.chemrev.6b00558 |
| [98] |
D. Geng, S. Yang, Y. Zhang, et al., Appl. Surf. Sci. 257 (2011) 9193-9198. DOI:10.1016/j.apsusc.2011.05.131 |
| [99] |
Y. Shao, S. Zhang, M.H. Engelhard, et al., J. Mater. Chem. 20 (2010) 7491-7496. DOI:10.1039/c0jm00782j |
| [100] |
Z. Liu, Z. Zhao, Y. Wang, et al., Adv. Mater. 29 (2017) 1606207.
|
| [101] |
L. Tao, Q. Wang, S. Dou, et al., Chem. Commun. (Camb.) 52 (2016) 2764-2767. DOI:10.1039/C5CC09173J |
| [102] |
J. Sun, S.E. Lowe, L. Zhang, et al., Angew. Chem. Int. Ed. 57 (2018) 16511-16515. DOI:10.1002/anie.201811573 |
| [103] |
H. Jiang, J. Gu, X. Zheng, et al., Energy Environ. Sci. 12 (2019) 322-333. DOI:10.1039/C8EE03276A |
| [104] |
H.W. Liang, Z.Y. Wu, L.F. Chen, C. Li, S.H. Yu, Nano Energy 11 (2015) 366-376. DOI:10.1016/j.nanoen.2014.11.008 |
| [105] |
X. Cui, S. Yang, X. Yan, et al., Adv. Funct. Mater. 26 (2016) 5708-5717. DOI:10.1002/adfm.201601492 |
| [106] |
R. Paul, F. Du, L. Dai, et al., Adv. Mater. 31 (2019) 1805598.
|
| [107] |
C. Wang, N.H. Xie, Y. Zhang, et al., Chem. Mater. 31 (2019) 1023-1029. DOI:10.1021/acs.chemmater.8b04572 |
| [108] |
H. Zhou, J. Zhang, I.S. Amiinu, et al., Phys. Chem. Chem. Phys. 18 (2016) 10392-10399. DOI:10.1039/C6CP00174B |
| [109] |
H. Wu, J. Geng, H. Ge, et al., Adv. Energy Mater. 6 (2016) 1600794.
|
| [110] |
M. Rana, K. Subramani, M. Sathish, U.K. Gautam, Carbon 114 (2017) 679-689. DOI:10.1016/j.carbon.2016.12.059 |
| [111] |
F. Pan, Z. Cao, Q. Zhao, H. Liang, J. Zhang, J. Power Sources 272 (2014) 8-15. DOI:10.1016/j.jpowsour.2014.07.180 |
| [112] |
S. Gao, X. Li, L. Li, X. Wei, Nano Energy 33 (2017) 334-342. DOI:10.1016/j.nanoen.2017.01.045 |
| [113] |
P. Fu, L. Zhou, L. Sun, B. Huang, Y. Yuan, RSC Adv. 7 (2017) 13383-13389. DOI:10.1039/C7RA00433H |
| [114] |
Y. Fang, H. Wang, H. Yu, F. Peng, Electrochim. Acta 213 (2016) 273-282. DOI:10.1016/j.electacta.2016.07.121 |
| [115] |
K.N. Chaudhari, M.Y. Song, J.S. Yu, Small 10 (2014) 2625-2636. DOI:10.1002/smll.201303831 |
| [116] |
M. Borghei, N. Laocharoen, E. Kibena-Põldsepp, et al., Appl. Catal. B 204 (2017) 394-402. DOI:10.1016/j.apcatb.2016.11.029 |
| [117] |
J. Deng, M. Li, Y. Wang, Green Chem. 18 (2016) 4824-4854. DOI:10.1039/C6GC01172A |
| [118] |
X. Peng, L. Zhang, Z. Chen, et al., Adv. Mater. 31 (2019) 1900341.
|
| [119] |
M. Zhou, C. Yang, K.Y. Chan, Adv. Energy Mater. 4 (2014) 1400840.
|
| [120] |
W. Niu, L. Li, X. Liu, et al., J. Am. Chem. Soc. 137 (2015) 5555-5562. DOI:10.1021/jacs.5b02027 |
| [121] |
Q.Y. Zhou, L. Zhao, X.L. Sui, et al., Chem. Asian J. 13 (2018) 3057-3062. DOI:10.1002/asia.201801134 |
| [122] |
D.H. Kwak, S.B. Han, D.H. Kim, J.E. Won, K.W. Park, Appl. Catal. B 238 (2018) 93-103. DOI:10.1016/j.apcatb.2018.07.013 |
| [123] |
C. Hu, J. Liu, J. Wang, et al., ACS Appl. Mater. Interfaces 10 (2018) 33124-33134. DOI:10.1021/acsami.8b07343 |
| [124] |
B.-C. Hu, Z.-Y. Wu, Q. S.-Chu, et al., Energy Environ. Sci. 11 (2018) 2208-2215. DOI:10.1039/C8EE00673C |
| [125] |
Z. Pei, H. Li, Y. Huang, et al., Energy Environ. Sci. 10 (2017) 742-749. DOI:10.1039/C6EE03265F |
| [126] |
M.H. Sun, S.Z. Huang, L.H. Chen, et al., Chem. Soc. Rev. 45 (2016) 3479-3563. DOI:10.1039/C6CS00135A |
| [127] |
Y. Zheng, Y. Jiao, Y. Zhu, et al., J. Am. Chem. Soc. 139 (2017) 3336-3339. DOI:10.1021/jacs.6b13100 |
| [128] |
Z. Wen, S. Ci, Y. Hou, J. Chen, Angew. Chem. Int. Ed. 53 (2014) 6496-6500. DOI:10.1002/anie.201402574 |
| [129] |
J. Sheng, L. Wang, L. Deng, et al., ACS Appl. Mater. Interfaces 10 (2018) 7191-7200. DOI:10.1021/acsami.8b00573 |
| [130] |
Y. Hu, J.O. Jensen, W. Zhang, et al., Angew. Chem. Int. Ed. 53 (2014) 3675-3679. DOI:10.1002/anie.201400358 |
| [131] |
A. Zitolo, V. Goellner, V. Armel, et al., Nat. Mater. 14 (2015) 937-942. DOI:10.1038/nmat4367 |
| [132] |
H. Shen, E. Gracia-Espino, J. Ma, et al., Angew. Chem. Int. Ed. 56 (2017) 13800-13804. DOI:10.1002/anie.201706602 |
| [133] |
Q. Jia, N. Ramaswamy, U. Tylus, et al., Nano Energy 29 (2016) 65-82. DOI:10.1016/j.nanoen.2016.03.025 |
| [134] |
J. Wang, L. Li, X. Chen, et al., Nano Res. 10 (2017) 2508-2518. DOI:10.1007/s12274-017-1455-0 |
| [135] |
F. Meng, H. Zhong, D. Bao, J. Yan, X. Zhang, J. Am. Chem. Soc. 138 (2016) 10226-10231. DOI:10.1021/jacs.6b05046 |
| [136] |
H. Jiang, Y. Liu, J. Hao, et al., ACS Sustain. Chem. Eng. 5 (2017) 5341-5350. DOI:10.1021/acssuschemeng.7b00655 |
| [137] |
J. Gao, N. Ma, Y. Zheng, et al., ChemCatChem 9 (2017) 1601-1609. DOI:10.1002/cctc.201601207 |
| [138] |
S. Wang, Q. He, C. Wang, et al., Small 14 (2018) 1800128.
|
| [139] |
R. Wang, T. Yan, L. Han, et al., J. Mater. Chem. A:Mater. Energy Sustain. 6 (2018) 5752-5761. DOI:10.1039/C8TA00439K |
| [140] |
Z. Liang, X. Fan, H. Lei, et al., Angew. Chem. Int. Ed. 57 (2018) 13187-13191. DOI:10.1002/anie.201807854 |
| [141] |
T. Feng, M. Zhang, Chem. Commun. (Camb.) 54 (2018) 11570-11573. DOI:10.1039/C8CC05959D |
| [142] |
J. Ban, G. Xu, L. Zhang, et al., Nanoscale 10 (2018) 9077-9086. DOI:10.1039/C8NR01457D |
| [143] |
L. Shang, H. Yu, X. Huang, et al., Adv. Mater. 28 (2016) 1668-1674. DOI:10.1002/adma.201505045 |
| [144] |
W. Yang, X. Liu, L. Chen, L. Liang, J. Jia, Chem. Commun. (Camb.) 53 (2017) 4034-4037. DOI:10.1039/C7CC01349C |
| [145] |
L. Chen, Y. Zhang, X. Liu, et al., Chem. Commun. (Camb.) 55 (2019) 5651-5654. DOI:10.1039/C9CC01705D |
| [146] |
L. Chen, Y. Zhang, X. Liu, et al., Carbon 151 (2019) 10-17. DOI:10.1016/j.carbon.2019.05.063 |
| [147] |
S. Li, B. Li, L. Ma, J. Yang, H. Xu, Chin. Chem. Lett. 28 (2017) 2159-2163. DOI:10.1016/j.cclet.2017.08.029 |
| [148] |
X. Chen, X. Zhen, H. Gong, et al., Chin. Chem. Lett. 30 (2019) 681-685. DOI:10.1016/j.cclet.2018.09.017 |
| [149] |
D.S. Su, G. Sun, Angew. Chem. Int. Ed. 50 (2011) 11570-11572. DOI:10.1002/anie.201106166 |
| [150] |
Y.J. Sa, D.J. Seo, J. Woo, et al., J. Am. Chem. Soc. 138 (2016) 15046-15056. DOI:10.1021/jacs.6b09470 |
| [151] |
Q. Lai, L. Zheng, Y. Liang, et al., ACS Catal. 7 (2017) 1655-1663. DOI:10.1021/acscatal.6b02966 |
| [152] |
G.A. Ferrero, K. Preuss, A. Marinovic, et al., ACS Nano 10 (2016) 5922-5932. DOI:10.1021/acsnano.6b01247 |
| [153] |
W.A. Saidi, J. Phys. Chem. Lett. 4 (2013) 4160-4165. DOI:10.1021/jz402090d |
| [154] |
L. Lai, J.R. Potts, D. Zhan, et al., Energy Environ. Sci. 5 (2012) 7936-7942. DOI:10.1039/c2ee21802j |
| [155] |
U. Tylus, Q. Jia, K. Strickland, et al., J. Phys. Chem. C 118 (2014) 8999-9008. DOI:10.1021/jp500781v |
| [156] |
G. Ren, L. Gao, C. Teng, et al., ACS Appl. Mater. Interfaces 10 (2018) 10778-10785. DOI:10.1021/acsami.7b16936 |
| [157] |
Z. Liu, F. Sun, L. Gu, et al., Adv. Energy Mater. 7 (2017) 1701154.
|
| [158] |
W.J. Jiang, L. Gu, L. Li, et al., J. Am. Chem. Soc. 138 (2016) 3570-3578. DOI:10.1021/jacs.6b00757 |
| [159] |
C. Guo, B. Wen, W. Liao, et al., J. Alloys. Compd. 686 (2016) 874-882. DOI:10.1016/j.jallcom.2016.06.243 |
| [160] |
M. Qiao, S.S. Meysami, G.A. Ferrero, et al., Adv. Funct. Mater. 28 (2018) 1707284.
|
| [161] |
L.C. Pardo Pérez, N.R. Sahraie, J. Melke, et al., Adv. Funct. Mater. 28 (2018) 1707551.
|
| [162] |
Y. Gao, L. Wang, G. Li, et al., Int. J. Hydrogen Energy 43 (2018) 7893-7902. DOI:10.1016/j.ijhydene.2018.03.043 |
| [163] |
J. Cao, X. Jia, M. Guo, et al., Sustain. Energy Fuels 2 (2018) 169-174. DOI:10.1039/C7SE00458C |
| [164] |
P. Chen, T. Zhou, L. Xing, et al., Angew. Chem. Int. Ed. 56 (2017) 610-614. DOI:10.1002/anie.201610119 |
| [165] |
W. Yang, Y. Zhang, X. Liu, L. Chen, J. Jia, Chem. Commun. (Camb.) 53 (2017) 12934-12937. DOI:10.1039/C7CC08008E |
| [166] |
K. Shen, X. Chen, J. Chen, Y. Li, ACS Catal. 6 (2016) 5887-5903. DOI:10.1021/acscatal.6b01222 |
| [167] |
A. Mahmood, W. Guo, H. Tabassum, R. Zou, Adv. Energy Mater. 6 (2016) 1600423.
|
| [168] |
Y. Ye, H. Li, F. Cai, et al., ACS Catal. 7 (2017) 7638-7646. DOI:10.1021/acscatal.7b02101 |
| [169] |
X. Wan, R. Wu, J. Deng, et al., J. Mater. Chem. A:Mater. Energy Sustain. 6 (2018) 3386-3390. DOI:10.1039/C7TA10022A |
| [170] |
S. Liu, Z. Wang, S. Zhou, et al., Adv. Mater. 29 (2017) 1700874.
|
| [171] |
H.M. Barkholtz, J. D.-Liu, Mater. Horiz. 4 (2017) 20-37. DOI:10.1039/C6MH00344C |
| [172] |
Z. Li, M. Shao, L. Zhou, et al., Adv. Mater. 28 (2016) 2337-2344. DOI:10.1002/adma.201505086 |
| [173] |
Y.V. Kaneti, J. Tang, R.R. Salunkhe, et al., Adv. Mater. 29 (2017) 1604898.
|
| [174] |
M. Zhang, Q. Dai, H. Zheng, M. Chen, L. Dai, Adv. Mater. 30 (2018) 1705431.
|
| [175] |
J. Guo, Y. Li, Y. Cheng, L. Dai, Z. Xiang, ACS Nano 11 (2017) 8379-8386. DOI:10.1021/acsnano.7b03807 |
| [176] |
J. Wei, Y. Liang, Y. Hu, et al., Angew. Chem. Int. Ed. 55 (2016) 1355-1359. DOI:10.1002/anie.201509024 |
| [177] |
F. Tang, H. Lei, S. Wang, H. Wang, Z. Jin, Nanoscale 9 (2017) 17364-17370. DOI:10.1039/C7NR06844A |
| [178] |
J. Park, H. Lee, Y.E. Bae, et al., ACS Appl. Mater. Interfaces 9 (2017) 28758-28765. DOI:10.1021/acsami.7b08786 |
| [179] |
Z. Li, Z. Zhuang, F. Lv, et al., Adv. Mater. 30 (2018) 1803220.
|
| [180] |
E. Li, F. Yang, Z. Wu, et al., Small 14 (2018) 1702827.
|
| [181] |
L. Cao, Z. Li, Y. Gu, et al., J. Mater. Chem. A:Mater. Energy Sustain. 5 (2017) 11340-11347. DOI:10.1039/C7TA03097E |
| [182] |
W. Yang, Y. Zhai, X. Yue, Y. Wang, J. Jia, Chem. Commun. (Camb.) 50 (2014) 11151-11153. |
| [183] |
M. Xiao, J. Zhu, L. Feng, C. Liu, W. Xing, Adv. Mater. 27 (2015) 2521-2527. DOI:10.1002/adma.201500262 |
| [184] |
G. Ren, X. Lu, Y. Li, et al., ACS Appl. Mater. Interfaces 8 (2016) 4118-4125. DOI:10.1021/acsami.5b11786 |
| [185] |
J. Li, S. Mao, Y. Hou, L. Lei, C. Yuan, ChemSusChem 11 (2018) 3292-3298. DOI:10.1002/cssc.201801084 |
| [186] |
W. Yang, X. Liu, X. Yue, J. Jia, S. Guo, J, . Am. Chem. Soc. 137 (2015) 1436-1439. DOI:10.1021/ja5129132 |
| [187] |
E.C. Tyo, S. Vajda, Nat. Nanotech. 10 (2015) 577-588. DOI:10.1038/nnano.2015.140 |
| [188] |
H. Yin, H. Tang, D. Wang, Y. Gao, Z. Tang, ACS Nano 6 (2012) 8288-8297. DOI:10.1021/nn302984x |
| [189] |
C. Zhu, S. Fu, Q. Shi, D. Du, Y. Lin, Angew. Chem. Int. Ed. 56 (2017) 13944-13960. DOI:10.1002/anie.201703864 |
| [190] |
Y. Peng, B. Lu, S. Chen, Adv. Mater. 30 (2018) 1801995.
|
| [191] |
J. Liu, ACS Catal. 7 (2016) 34-59. |
| [192] |
D. Ji, L. Fan, L. Li, et al., Adv. Mater. 31 (2019) 1808267.
|
| [193] |
B.Q. Li, C.X. Zhao, S. Chen, et al., Adv. Mater. 31 (2019) 1900592.
|
| [194] |
Y. He, S. Hwang, D.A. Cullen, et al., Energy Environ. Sci. 12 (2019) 250-260. DOI:10.1039/C8EE02694G |
| [195] |
Z. Miao, X. Wang, M.C. Tsai, et al., Adv. Energy Mater. 8 (2018) 1801226.
|
| [196] |
Y. Chen, Z. Li, Y. Zhu, et al., Adv. Mater. 31 (2019) 1806312.
|
| [197] |
M. Zhang, Y.G. Wang, W. Chen, et al., J. Am. Chem. Soc. 139 (2017) 10976-10979. DOI:10.1021/jacs.7b05372 |
| [198] |
C. Zhu, Q. Shi, B.Z. Xu, et al., Adv. Energy Mater. 8 (2018) 1801956.
|
| [199] |
Y. Han, Y. Wang, R. Xu, et al., Energy Environ. Sci. 11 (2018) 2348-2352. DOI:10.1039/C8EE01481G |
| [200] |
G. Zhang, G. Wang, Y. Liu, et al., J. Am. Chem. Soc. 138 (2016) 14686-14693. DOI:10.1021/jacs.6b08491 |
| [201] |
K.P. Singh, E.J. Bae, J.S. Yu, J. Am. Chem. Soc. 137 (2015) 3165-3168. DOI:10.1021/ja511759u |
| [202] |
X. Qiao, J. Jin, H. Fan, Y. Li, S. Liao, J. Mater. Chem. A:Mater. Energy Sustain. 5 (2017) 12354-12360. DOI:10.1039/C7TA00993C |
| [203] |
H. Hu, L. Han, M. Yu, Z. Wang, X.W. Lou, Energy Environ. Sci. 9 (2016) 107-111. DOI:10.1039/C5EE02903A |
| [204] |
H. Yuan, L. Kong, T. Li, Q. Zhang, Chin. Chem. Lett. 28 (2017) 2180-2194. DOI:10.1016/j.cclet.2017.11.038 |
| [205] |
R. Zhang, C. Zhang, W. Chen, J. Mater. Chem. A:Mater. Energy Sustain. 4 (2016) 18723-18729. DOI:10.1039/C6TA08363C |
| [206] |
X. Xu, C. Shi, R. Chen, T. Chen, RSC Adv. 7 (2017) 22263-22269. DOI:10.1039/C7RA02349A |
| [207] |
G. Wang, J. Li, M. Liu, L. Du, S. Liao, ACS Appl. Mater. Interfaces 10 (2018) 32133-32141. DOI:10.1021/acsami.8b10373 |
| [208] |
L. Hu, F. Yu, H. Yuan, et al., Chin. Chem. Lett. 30 (2019) 624-629. DOI:10.1016/j.cclet.2018.10.039 |
| [209] |
Y. Lin, L. Yang, Y. Zhang, et al., Adv. Energy Mater. 8 (2018) 1703623.
|
| [210] |
H. Li, Q. Li, P. Wen, et al., Adv. Mater. 30 (2018) 1705796.
|
| [211] |
C. Han, Q. Li, D. Wang, et al., Small 14 (2018) 1703642.
|
| [212] |
Q.L. Zhu, W. Xia, T. Akita, R. Zou, Q. Xu, Adv. Mater. 28 (2016) 6391-6398. DOI:10.1002/adma.201600979 |
| [213] |
S. Dou, L. Tao, J. Huo, S. Wang, L. Dai, Energy Environ. Sci. 9 (2016) 1320-1326. DOI:10.1039/C6EE00054A |
| [214] |
S. Guo, S. Zhang, L. Wu, S. Sun, Angew. Chem. Int. Ed. 51 (2012) 11770-11773. DOI:10.1002/anie.201206152 |
| [215] |
C. Guan, A. Sumboja, H. Wu, et al., Adv. Mater. 29 (2017) 1704117.
|
| [216] |
J. Zhao, C. Li, R. Liu, Nanoscale 10 (2018) 5882-5887. DOI:10.1039/C7NR09185K |
| [217] |
H. Zhang, J. Xu, Y. Jin, et al., Chemistry 24 (2018) 14522-14530. DOI:10.1002/chem.201802898 |
| [218] |
X. Xu, C. Shi, Q. Li, R. Chen, T. Chen, RSC Adv. 7 (2017) 14382-14388. DOI:10.1039/C6RA27826D |
| [219] |
H. Tang, W. Chen, J. Wang, et al., Small 14 (2018) 1703459.
|
| [220] |
X. Li, F. Dong, N. Xu, et al., ACS Appl. Mater. Interfaces 10 (2018) 15591-15601. DOI:10.1021/acsami.7b18684 |
| [221] |
J. Fang, L. Hu, M. Wang, et al., Mater. Lett. 218 (2018) 36-39. DOI:10.1016/j.matlet.2018.01.061 |
| [222] |
A. Aijaz, J. Masa, C. Rosler, et al., Angew. Chem. Int. Ed. 55 (2016) 4087-4091. DOI:10.1002/anie.201509382 |
| [223] |
L. Chen, W. Yang, X. Liu, et al., Nanotechnology 30 (2019) 075402.
|
| [224] |
X. Han, W. Zhang, X. Ma, et al., Adv. Mater. 31 (2019) 1808281.
|
| [225] |
T. Zhou, W. Xu, N. Zhang, et al., Adv. Mater. 31 (2019) 1807468.
|
 2020, Vol. 31
2020, Vol. 31