Hydrogen (H2), has attracted extensive attention as it is a clean and renewable fuel, and can be used as an important industrial reagent for the production of ammonia and synthetic gas [1-3]. There are several methods developed for the production of clean H2 fuel, which encompass electrochemical (EC) and photoelectrochemical (PEC) water splitting reactions [2, 4]. Electrochemical water splitting comprises a system where the potential to drive the water oxidation-reduction between both the electrodes (theoretically~1.23 V) is supplied by an external power source such as a battery. Compared to EC driven water splitting, PEC water splitting is a more effective method, as the entire or fraction of the voltage that is required to drive these reaction is harnessed by the absorption of incoming photons of the solar radiation [4]. In either case, efficient large-scale production of H2 at the cathode is hindered by the sluggish O2 evolution reaction (OER) at the anode. In order to facilitate the OER, to date, various earth-abundant catalysts including carbon based catalysts and transition metal oxides have been developed to replace noble metal-based catalysts [5-8]. Among these, transition metal oxides have been investigated as low-cost alternative catalysts for OER due to isoelectronic properties and superior stability. As mentioned before, some transition metal oxides resemble semiconductor like properties in the PEC reaction, where they absorb the incoming solar radiation to generate photo-excited charges and further split water for the production of H2 fuel. Until now, transition metal oxides, such as Co3O4, Mn3O4, and NiO, have demonstrated to possess superior electrocatalytic ability for OER [9, 10], while transition metal oxides such as TiO2, ZnO, α-Fe2O3, and WO3 have been identified as promising light-absorbing semiconductor materials for PEC water splitting [4]. Accordingly, different processing methods have been developed for the fabrication of transition metal oxides, which include sputtering, chemical vapour deposition (CVD), electrode-position, anodic oxidization, sol-gel and hydrothermal methods [11, 12]. However, many of these fabrication methods require multi-steps, are expensive and have limited scalability. In order to meet the requirement for batch fabrication, it is imperative to develop an effective method to fabricate transition metal oxides, which is rapid, reproducible and scalable.
Flame spray pyrolysis (FSP) is one such method that provides avenues for large-scale production and has been widely employed to produce carbon black, fumed silica, and P25 on a commercial scale [13]. These FSP-made powders can be tuned between a few nanometer to a micrometer size particles, with a precise control on the size distribution, thus having high surface areas which is a an essential criteria for applications ranging in industries pertaining to paint, catalysis, and also as additives in production of rubber [13]. The above mentioned advantages of FSP method has led to an extensive research on the development of FSP reactor, under-stating the role of precursor chemistry on the morphology and structure of the final products. These findings have been summarized in several recent review papers [14-18]. More recently, FSP has also been developed for the fabrication of porous network of films, consisting of nanoparticles with tunable porosity, demonstrating excellent performance in chemical sensors, photo-detectors, and solar cells [19, 20]. Although FSP-made porous films are suitable in the above applications (vide supra), the brittle nature of these films pose a major challenge especially in harsh aqueous solutions utilized in water oxidation-reduction reactions. FSP-made powders can also be casted or dropped into films; however, these films are relatively dense with non-uniform coverage arising from the wetting processes and solvent evapora-tion, resulting in a lack of three-dimensional hierarchy. Recently, our group has made progress on the direct FSP fabrication of robust transition metal oxide films on the solid substrates, which show promising performance either as efficient catalysts for EC water splitting or as semiconductor materials for PEC water splitting.
In this short review, we introduce the use of the FSP technique for the direct fabrication of various transition metal oxide films, and perform a systematic investigation of their recent applications in EC/PEC water splitting. We also provide a perspective on the design of functional FSP-fabricated transitional metal oxide films for future energy storage and conversion.
2. FSP technique for the fabrication of direct and robust transition metal oxide filmsA schematic of the FSP setup used for the one-step fabrication of transition metal oxide films is shown in Fig. 1. The apparatus consists of a syringe pump, a gas-assisted spray nozzle with six supporting CH4/O2 flamelets, and a chamber with filter paper which is connected with a vacuum pump. In a typical FSP synthesis process, the precursor is premixed with a liquid fuel and fed into the nozzle by a syringe pump, and then atomized by an oxygen dispersion gas. The atomized precursor solution is ignited by the surrounding premixed CH4/O2 flamelets leading to a continuous spray flame. Subsequently, the combustion of the precursor results in the formation of a super-saturated vapor of the target metal atoms, which nucleates forming the first stable clusters, which then grow into nanoparticles and larger fractal-like agglomerates. This aerosol can be collected either as powder on filters or as film on a solid substrate. Because of the highly exothermic nature of FSP liquid precursor combustion, the flame temperature can reach up to 1000 ℃. Therefore, the FSP-made porous films are often fabricated at an optimal height above burner (HAB) to induce a certain amount of sintering of the nanoparticles, and the substrate is water-cooled to avoid damage. Under these experimental conditions, the FSP-made films have tunable porosity. At HAB of 10 cm and above, the FSP-made films have very high porosity and large surface areas, but suffer of very poor mechanical adhesion with the underlying substrate [19]. Although post-thermal treatment have been applied to alleviate this issue, these films still cannot endure the repetitive measurements during the gas evolution reactions on their surface, nor the strong capillary forces acting on the pore formed between the nanograin boundary [21, 22].

|
Download:
|
| Fig. 1. Schematic representation of the one-step fabrication of (photo)electrodes by corresponding cross-sectional SEM images (b, d, f). P stands for porosity. | |
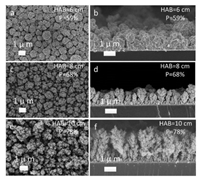
To extend the use FSP for the fabrication of EC/PEC water splitting electrodes, the process conditions need to be optimized. The substrate needs be in closer proximity to the flame for the deposition of the nanoparticle aerosol and achieve in-situ sintering to form the robust morphologies. At the same time, the substrate must also endure high temperature deposition without thermal damage. Recently, our group determined the HAB and deposition times required for the direct fabrication of robust FSP-made WO3 and BiVO4 films on fluorine-doped tin oxide (FTO) glass [23, 24]. As shown in Fig. 2, the morphology of FSP-made WO3 films can be tuned from tree-like structure at HAB of 10 cm to cauliflower-like structure at HAB of 6 cm. Accordingly, the porosity of these WO3 films have also been changed from 78% to 68%, then to 59% at HAB of 10 cm, 8 cm, 6 cm, respectively. These findings enable the use of FSP as a powerful technique for the direct fabrication of robust films with tunable morphology and porosity.
|
Download:
|
| Fig. 2. Morphological characterization of one-step FSP-made WO3 films with representative top-views at HAB of 6 cm (a), 8 cm (c) and 10 cm (e) and their lame spray pyrolysis. Reproduced with permission [23]. Copyright 2018, Wiley. | |

|
Download:
|
| Fig. 3. EC performance of FSP-made Co3O4 films (a) and PEC performance of FSP-made WO3 films (b) and BiVO4 films (c). Reproduced with permission [23, 24, 26]. Copyright 2016, 2018, 2019, Wiley. | |
3. FSP-made transition metal oxide films for EC and PEC water splitting
As efficient EC catalysts, precious metals have been adopted for OER in water splitting. However, large-scale production has been limited due to their scarcity, leading to explore earth-abundant transition metal oxides as suitable EC catalysts for water oxidation [5]. Amongst them, FSP-made manganese oxide has been confirmed to possess substantially high catalytic activity for OER, with low overpotentials of 0.35 V and reaching current density of 10 mA/cm2, demonstrating the feasibility of FSP as a promising method for rapid and scalable production of earth-abundant nanocatalysts with enhanced catalytic activity for water oxidation [25]. Similarly, FSP-made Co3O4 catalysts demonstrated an outstanding electrochemical and mechanical integrity during 1000 voltammetric cycles and 12 h of constant current catalytic tests, with a remarkable mass-weighted water oxidation activity of 2070–2350 A/gCo3O4 and per-metal turnover frequency of 0.38-0.62 s-1 at an overpotential of 400 mV in 1 mol/L NaOH aqueous solution (Fig. 3a) [26]. More importantly, the robust sub-monolayers of Co3O4 is highly transparent with more than 94% light transmission at a wavelength of 500 nm, which makes it an ideal co-catalyst for the fabrication of efficient PEC devices without any major optical losses. As a demonstration, Co3O4-decorated GaN nanowires photoanodes revealed significantly reduced onset overpotentials, improved photocurrent density and photostability compared to bare GaN ones [26]. This provides an effective solution for solving the parasitic light losses at high co-catalysts mass loadings on photoelectrodes.
In addition to EC catalysts, some FSP-made transition metal oxides have also be used for the fabrication of photoelectrodes in PEC water splitting. As a well-established and scalable method, FSP has been adopted for the production of various transition metal oxide powders [15]. Accordingly, some FSP-made semiconductor powders can also be fabricated as films on FTO substrates as photoelectrodes for PEC water splitting or dye-sensitized solar cells. For example, FSP-made titania powders can be fabricated as photoanodes using doctor-blade method and the resulting photo-anodes demonstrate superiority in PEC water splitting with respect to commercial P25 titania [27]. FSP-made CuO powders were spin coated on ITO substrates and served as photocathodes for PEC water splitting, resulting in 1.20 mA/cm2 at applied voltage of -0.55 V vs. Ag/AgCl in 1 mol/L KOH electrolyte under 1 sun (100 mW/cm2, AM1.5 G) illumination [28]. After doping with lithium, the electrical conductivity of these FSP-made CuO powders increased by almost two orders of magnitude and the current density of the spin-coated CuO films reached up to 1.69 mA/cm2 under the same experimental condition [29]. FSP-made BiVO4 powders can also be fabricated as visible-light-active photoelectrodes by doctor-blading. It was shown that the current density of BiVO4 photoelectrodes increased by twofold after aqueous treatment with additional precursors, but the current density was still quite low (3.3 μA/cm2 to 3.8 μA/cm2) [30].
To increase the mechanical adhesion and further facilitate the photo-excited charges transfer from the semiconductor photo-electrodes to the underlying conductive FTO substrates, our group recently have developed a one-step FSP methodology to fabricate robust photoelectrodes for PEC water splitting [23]. Through optimizing the HAB and the deposition time, it was found that 10 s-deposited WO3 films with an HAB of 6 cm displayed the best performance; with a current density of 0.91 mA/cm2 at 1.23 V vs. reversible hydrogen electrode (RHE) under simulated 1 sun irradiation (Fig. 3b). It was also shown that FSP-made WO3 photoanodes also possess good mechanical stability with only slight degradation after 1 h of continuous test. In comparison, the current density of these FSP-made WO3 photoanodes is 2 to 3 times higher than that of doctor-blade fabricated electrodes [23], suggesting a more functional morphology. As an extended research work, FSP-made BiVO4 photoanodes also displayed good perfor-mance, with 1.5 mA/cm2 for sulphite oxidation and ca. 1 mA/cm2 for water oxidation with a FeOOH/NiOOH co-catalyst at 1.0 V vs. RHE under simulated 1 sun illumination (Fig. 3c) [24]. The performance of these one-step FSP-made BiVO4 photoanodes is significantly improved over that of the aforementioned doctor-bladed electrodes [30]. This study also revealed two competitive factors, viz. accessible surface area and carrier conductivity through the grain boundaries, play important role in determining the PEC performance. Although the performance of direct FSP-made photoelectrodes has been greatly improved compared with those using FSP-made powders, there are still some post treat-ments which could be performed to tune the structure, such as morphology, crystallinity, and preferential orientation, to further enhance their PEC performance in water splitting. For example, the PEC performance of hematite-based photoanodes is normally limited by the low charge separation efficiency and poor charge transport of the minaority charge carriers [31]. Considering this, Sn doped hematite photoanodes can be directly fabricated by FSP, which improve conductivity and also improve the directional transport of the charge carriers. Through post physically- and chemically-induced morphological and structural tuning proce-dures, a 24-fold enhancement in the photocurrent density for water oxidation (1 mol/L NaOH) at 1.23 V vs. RHE under simulated 1 sun irradiation has been achieved [32]. Overall the above findings showcase the promising use of one-step FSP for the rapid and scalable fabrication of efficient EC catalysts and PEC photo-electrodes for water splitting.
4. Conclusion and outlookAlthough the fabrication of FSP-made nanostructured hierar-chical films has made great progress in recent years, their applications in energy storage and conversion has not been extensively investigated in comparison to that of FSP-made powders and other wet-chemical synthesized films. Therefore, it is a requirement to develop methodologies for the design and fabrication of functional FSP-made films with tailored structures and properties to satisfy the requirements in EC and PEC water splitting and other energy storage and conversion applications. Research directions include:
(1)FSP is a powerful technique for the fabrication of various doped materials, such as the explored Sn doped hematite and Li doped CuO [27, 30]. This advantage should be explored for the fabrication of FSP-made films with improved optical and electronic properties.
(2)The twin-nozzle FSP system fed with two different precursors can be used for the fabrication of heterojunction nanostructures. Two flame nozzles with an optimal angle can uniformly mix the different aerosols together and further deposited on solid substrates as heterojunction films. Using this design, various heterojunction nanostructures such as n-n, p-p, p-n, even metal-modified transition metal oxides can be directly fabricated, providing more choice for the designing high efficient EC catalysts or photoelectrodes for water splitting.
(3)More complicated transition metal oxides such as binary and ternary metal oxides, including CuBi2O4, Fe2TiO5, BiCu2VO6, can also be fabricated as new EC catalysts or PEC photo-electrodes for water splitting, providing an insight for the broad exploration of other mixed-metal oxide combinations.
These opportunities may enable the use of FSP as a powerful technique for the direct, rapid and scalable fabrication of robust and functional films on solid substrates with the improved performance, thus paving the path for applications in EC/PEC water splitting and other applications in energy storage and conversion.
AcknowledgmentsA. Tricoli gratefully acknowledges the support of Australian Research Council (ARC) DP150101939,ARC DE160100569, Westpac 2016 Research Fellowship, and the ActewAGL Endowment Fund.
| [1] | |
| [2] | |
| [3] | |
| [4] |
M.G. Walter, E.L. Warren, J.R. McKone, et al., Chem. Rev. 110 (2010) 6446-6473. DOI:10.1021/cr1002326 |
| [5] |
I. Roger, M.A. Shipman, M.D. Symes, Nat. Rev. Chem. 1 (2017) 0003.
|
| [6] |
Z. Zhou, F. He, Y. Shen, et al., Chem. Commun. 53 (2017) 2044-2047. DOI:10.1039/C6CC09442B |
| [7] |
H. Mei, M. Yang, Y. Shen, et al., Carbon 144 (2019) 312-320. DOI:10.1016/j.carbon.2018.12.051 |
| [8] |
Z. Zhou, Y. Zhang, Y. Shen, S. Liu, Y. Zhang, Chem. Soc. Rev. 47 (2018) 2298-2321. DOI:10.1039/C7CS00840F |
| [9] |
C.C.L. McCrory, S. Jung, J.C. Peters, T.F. Jaramillo, J. Am. Chem. Soc. 135 (2013) 16977-16987. DOI:10.1021/ja407115p |
| [10] |
M. Tahir, L. Pan, F. Idrees, et al., Nano Energy 37 (2017) 136-157. DOI:10.1016/j.nanoen.2017.05.022 |
| [11] |
A. Kudo, Y. Miseki, Chem. Soc. Rev. 38 (2009) 253-278. DOI:10.1039/B800489G |
| [12] |
F.E. Osterloh, Chem. Soc. Rev. 42 (2013) 2294-2320. DOI:10.1039/C2CS35266D |
| [13] |
H.K. Kammler, L. Mädler, S.E. Pratsinis, Chem. Eng. Technol. 24 (2001) 583-596. DOI:10.1002/1521-4125(200106)24:6<583::AID-CEAT583>3.0.CO;2-H |
| [14] |
L. Mädler, H.K. Kammler, R. Mueller, S.E. Pratsinis, J. Aerosol Sci. 33 (2002) 369-389. DOI:10.1016/S0021-8502(01)00159-8 |
| [15] |
R. Strobel, S.E. Pratsinis, J. Mater. Chem. 17 (2007) 4743-4756. DOI:10.1039/b711652g |
| [16] |
W.Y. Teoh, Materials 6 (2013) 3194.
|
| [17] |
W.Y. Teoh, R. Amal, L. Mädler, Nanoscale 2 (2010) 1324-1347. DOI:10.1039/c0nr00017e |
| [18] |
S. Li, Y. Ren, P. Biswas, S.D. Tse, Prog. Energy Combust. Sci. 55 (2016) 1-59. DOI:10.1016/j.pecs.2016.04.002 |
| [19] |
A. Tricoli, M. Righettoni, A. Teleki, Angew. Chem. Int. Ed. 49 (2010) 7632-7659. DOI:10.1002/anie.200903801 |
| [20] |
N. Nasiri, R. Bo, F. Wang, L. Fu, A. Tricoli, Adv. Mater. 27 (2015) 4336-4343. DOI:10.1002/adma.201501517 |
| [21] |
A. Tricoli, M. Graf, F. Mayer, et al., Adv. Mater. 20 (2008) 3005-3010. DOI:10.1002/adma.200701844 |
| [22] |
A. Tricoli, N. Nasiri, H. Chen, A.S. Wallerand, M. Righettoni, Sol. Energy 136 (2016) 553-559. DOI:10.1016/j.solener.2016.07.024 |
| [23] |
H. Chen, R. Bo, T. Tran-Phu, G. Liu, A. Tricoli, ChemPlusChem 83 (2018) 569-576. DOI:10.1002/cplu.201800061 |
| [24] |
T. Tran-Phu, H. Chen, R. Bo, et al., Energy Technol. 7 (2019) 1801052.
|
| [25] |
G. Liu, J. Hall, N. Nasiri, et al., ChemSusChem 8 (2015) 4162-4171. DOI:10.1002/cssc.201500704 |
| [26] |
G. Liu, S.K. Karuturi, A.N. Simonov, et al., Adv. Energy Mater. 6 (2016) 1600697.
|
| [27] |
I. Tantis, M.V. Dozzi, L.G. Bettini, et al., Appl. Catal. B 182 (2016) 369-374. DOI:10.1016/j.apcatb.2015.09.040 |
| [28] |
C.Y. Chiang, K. Aroh, N. Franson, et al., Int. J. Hydrogen Energy 36 (2011) 15519-15526. DOI:10.1016/j.ijhydene.2011.09.041 |
| [29] |
C.Y. Chiang, Y. Shin, S. Ehrman, J. Electrochem. Soc. 159 (2011) B227-B231.
|
| [30] |
Y.K. Kho, W.Y. Teoh, A. Iwase, et al., ACS Appl. Mater. Interfaces 3 (2011) 1997-2004. DOI:10.1021/am200247y |
| [31] |
A.G. Tamirat, J. Rick, A.A. Dubale, W.N. Su, B.J. Hwang, Nanoscale Horiz. 1 (2016) 243-267. DOI:10.1039/C5NH00098J |
| [32] |
G. Liu, S.K. Karuturi, H. Chen, et al., Nano Energy 53 (2018) 745-752. DOI:10.1016/j.nanoen.2018.09.048 |
 2020, Vol. 31
2020, Vol. 31 

