b MOE Key Laboratory for Analytical Science of Food Safety and Biology, Fujian Provincial Key Laboratory of Analysis and Detection for Food Safety, College of Chemistry, Fuzhou University, Fuzhou 350116, China
Currently, most of the analytical methods depend largely on laboratory-based analytical techniques that require expensive and bulky equipment, including liquid chromatography coupled to mass spectrometry (LC–MS), gas chromatography coupled to mass spectrometry (GC–MS), and others techniques. The employment of these huge analytical instruments for the detection of various targets, such as small organic molecules, metal ions, biomolecules, could potentially incur costly tests. Meanwhile, they require relatively time-consuming and complicated sample pretreatment processes (e.g., digestion or clean-up steps), complicated equipment operations, trained personnel, and specific consumables, finally lengthen the detection process, and lead to the potential loss of the analyte [1]. Additionally, sample transports are also needed, as experiments cannot be performed outside specialized labora-tories [2].
The miniaturized analytical system, with special advantages of portability and low cost, can be used for applications outside the laboratory environment to overcome the drawbacks of conventional analytical procedures [2]. Related techniques have been developed to reduce total analytical time or increase the extraction efficiency, such as supercritical fluid extraction (SFE) [3]. Now, more attention has been paid to miniaturized analytical devices as the increasing requirements for point-of-care testing (POCT) [4].
Miniaturized electrochemical (MEC) sensors, which are typical miniaturized analytical devices, have been applied for the detection of trace amounts of target, including small organic molecules [5, 6], metal ions [7], and biomolecules [8], through measuring changes of the electrochemical signal, such as current, voltage, potential or impedance, due to the oxidation/reduction of chemical/biological molecules with the help of electrodes and electrochemical units [9]. Generally, electrodes are modified to improve the selectivity of sensors mostly by the conjugation of specific recognition elements, such as aptamers, antibodies, and receptors of interest [10].
MEC sensors systems offer the advantages of simple instrumentation, ease of use, high sensitivity and selectivity, minimal sample pretreatment, short analysis time, portability, and low-cost [11, 12]. Currently, considerable research efforts have been made to develop different MEC sensors for POCT use to analyze trace amounts of target in various fields, including health care [13, 14], food safety and environmental monitoring, owing to excellent advantages of electrochemical (EC) technologies [15].
Herein, we focus on the latest development of various types of EC sensors and their potential in miniaturization, and their application in POCT will be discussed in detail. Furthermore, the future perspectives, opportunities, and challenges in this field will also be discussed.
2. Classification of miniaturized electrochemical system 2.1. DNA-based miniaturized electrochemical sensorDNA, or aptamer, is an artificial nucleic acid or peptide screened by the systematic evolution of ligands by exponential enrichment (SELEX) [16], which can bind with targets ranging from proteins, cells, and amino acids to organic and inorganic ions with high affinity and specificity [17]. It has been broadly employed to fabricate MEC aptasensors as an excellent recognition element or functional unit. Aptamer-based MEC sensors have exhibited significant advantages over other analytical methods, such as having high sensitivity, satisfactory selectivity, easy fabrication, and miniaturization under a broad array of conditions [18]. Therefore, MEC aptasensors have great potential in making sequence-specific information accessible and miniaturization, which is of great significance in many fields especially in clinical, environmental, and food analysis [19]. Generally, MEC aptasensors can be divided into two categories, including immobilization MEC aptasensor and immobilization free MEC aptasensor. The two kinds of MEC aptasensor are summarized.
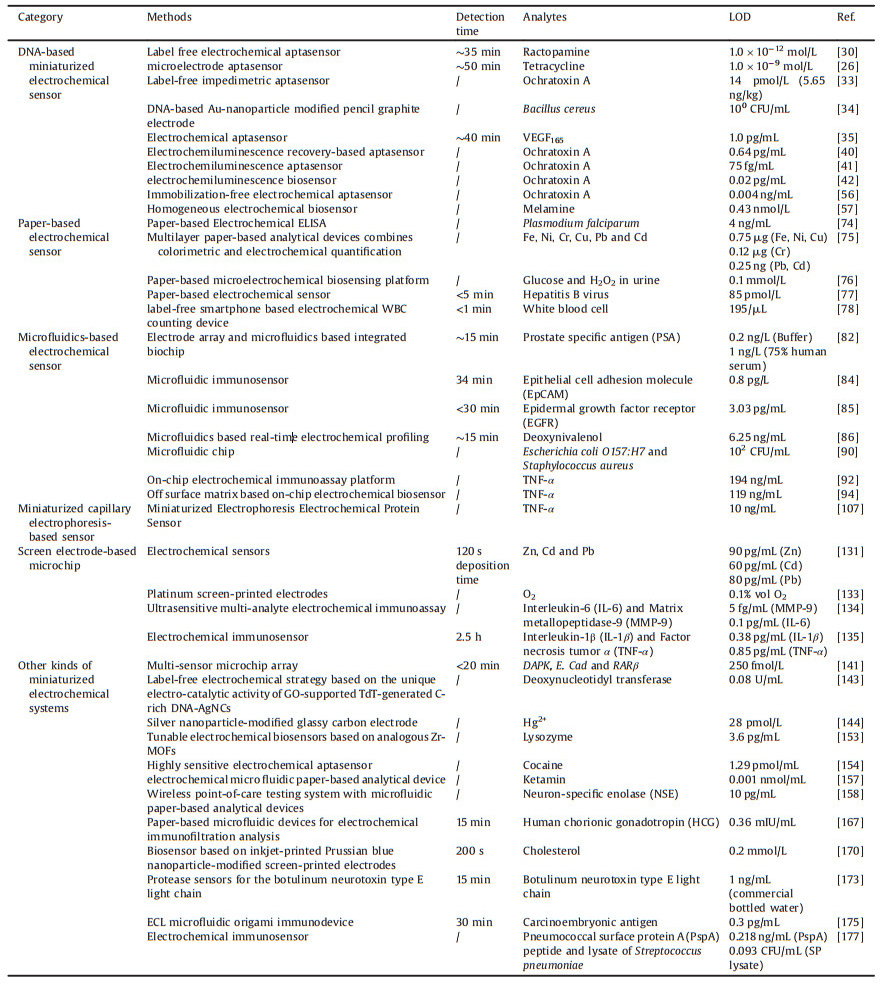
2.1.1. Immobilization miniaturized electrochemical sensorTraditionally, the EC aptasensor is fabricated via a DNA-modified electrode, including a glass carbon electrode [20], an indium tin oxide (ITO) electrode [21], a silicon oxide electrode [22], and a noble metal electrode [23]. The modified methods involve physical adsorption, electro polymeric membrane adsorption, self-assembly, and chemical bridging. As the ideal combination of electrochemical techniques and aptamers, the MEC aptasensors feature high sensitivity and selectivity, low cost, and miniaturized electrochemical devices, and thus have garnered significant attention for bioassays [24, 25]. Therefore, many excellent MEC aptasensors based on aptamer immobilization have been reported for the detection of small molecules [26, 27], metal ions [28], and biomolecules [29]. Our group developed a label-free MEC aptasensor for the ultrasensitive detection of ractopamine (RAC)[30]. More specifically, a special immobilization media consisting of gold nanoparticles/poly dimethyl diallyl ammonium chloride-graphene composite (AuNPs/PDDA-GN) was utilized to improve the conductivity and performance of the biosensor (Fig. 1). The RAC aptamer was attached on AuNPs of the composite membrane via an Au-S bond. The limit of detection (LOD) was 1.0×10-12 mol/L. Particularly, the generality of the fabrication approach of the electrochemical aptasensor was highlighted with a subsequent example for illegal drug detection via the aptamer identification.
|
Download:
|
| Fig. 1. Schematic illustration of the aptasensor fabrication process. Copied with permission [30]. Copyright 2015, Elsevier B.V. | |
It is very important for MEC sensors to evaluate the analytical performance, including robustness and specificity, in a real and complex sample matrix. However, some EC aptasensors, especially MEC aptasensors based on electrochemical impedance spectroscopy (EIS), are susceptible to nonspecific binding of interferents on the electrode surface, resulting in nonspecific electrochemical signal changes, which can affect the detection results [31]. With the remarkable achievements in nanotechnology, nano-material-based EC signal amplifications have great potential in miniaturi-zation and improving both sensitivity and selectivity for MEC aptasensors [32]. Rivas et al. developed a novel IrO2 nano-particles-based nanostructured platform to improve the analytical perfor-mance of EIS aptasensors [33]. Lower matrix interferences were achieved during the detection of ochratoxin A (OTA) in white wine samples, and the results illustrated the availability of the sensing system in analyzing real samples. Izadi et al. built an EIS-based MEC sensor with a gold-nano-particles-modified pencil graphite electrode for the detection of Bacillus cereus [34]. The as-prepared biosensor exhibited high accuracy to recognize nonhemolytic enterotoxin (nhe) A (nheA gene), which is the most prevalent marker in all subspecies of Bacillus cereus in real samples like milk and infant formula. Tabrizi et al. successfully synthesized ordered mesoporous carbon-gold nanocomposites that were modified on a screen printed electrode to construct an EIS aptasensor. The EIS aptasensor was then applied for the determination of VEGF165 in a serum sample of a lung cancer patient [35], and the recovery of target analytes was about 97.1%. The concentration of VEGF165 in serum sample was 712.2 pg/mL, and the result was satisfactory with the testing results of a standard enzyme-linked immunosor-bent assay (ELISA) value in a local hospital (733.5 pg/mL). These achievements of the developed method represented a huge potential for various diagnostic applications.
Baseline drift is also an important factor that affects the accuracy of MEC sensors. To overcome the disadvantage of baseline drift when the aptasensor was adopted for a complex sample matrix, Du et al. used an analogous approach to correct for sensor-to-sensor fabrication variability in MEC aptasensors [36]. Li et al. presented a dual-reporter approach to perform a correction of MEC aptasensor baseline drift [37]. The approach incorporated two redox reporters (methylene blue and anthraquinone) on the aptamer: One reporter served as the target-responsive sensor, and the other one, reporting at a distinct, non-overlapping redox potential, served as a drift correcting reference. Taking the difference in their relative signals largely eliminated the baseline drift observed for these sensors in flowing, undiluted whole blood, reducing drift of up to 50% to less than 2% over many hours of continuous operation under these challenging conditions.
Electrochemiluminescence (ECL) has also been paid great attention as the instruments for ECL detection are simple, cheap, and flexible [38]. Meanwhile, ECL is highly sensitive and flexible. ECL can combine with a specific aptamer to fabricate high performance ECL aptasensors with the promising potential of minimization and application in POCT [39]. For example, Yang et al. reported a highly sensitive ECL aptasensor for the detection of OTA on the basis of the ECL recovery of the quantum dot and exonuclease-catalyzed target recycling amplification [40]. Subsequently, they presented a highly sensitive ECL strategy for the detection of OTA through the target-induced autonomous disassembly of the aptamer-DNAzyme super sandwich nanostructures [41]. In this study, the presence of the OTA and the exonuclease resulted in the autonomous disassembly of the nanostructures and cyclic reuse of OTA, leading to the efficient recovery of the ECL emission and highly sensitive detection of OTA. Our group proposed a signal-on ECL aptasensor for the determination of OTA via the signal amplification strategy of hyperbranched rolling circle amplification (HRCA) [42]. The LOD of the developed method based on HRCA was achieved as low as 0.02 pg/mL. In addition, we developed an ultrasensitive ECL aptasensor for the detection of protein (thrombin as an example) and DNA from Bacillus subtilis based on HRCA [43, 44].
2.1.2. Immobilization-free miniaturized electrochemical sensorAs DNA immobilization process is usually required in the immobilization MEC sensors, the immobilization procedures are often considered to be complicated, laborious, and time-consum-ing. Hence, it is necessary to develop new electrochemical strategies which are immobilization-free and straightforward to use. The MEC biosensing based on an immobilization-free strategy was first reported by the Xuan group [45]. An ITO electrode was applied as the working electrode. The surface of the ITO electrode was negatively charged after simple treatment, and it could repel DNA because the molecular skeleton of DNA contains negatively charged phosphate. In view of these characteristics, many simple but sensitive homogeneous MEC biosensors have been developed for the detection of diverse targets [46-49].
DNA hybridization and target recognizing processes all occur in homogeneous solution phase, with the advantages of simple operation, rapid response, and improved reaction efficiency. However, the electrochemical response of the immobilization-free MEC aptasensor depends on the diffusion of electro-active molecules from the solution phase to the surface of the electrode. Because of this property of being diffusion controlled, the immobilization-free electrochemical method often exhibited a lower sensitivity compared with the immobilization-required assays [50]. To overcome the disadvantage and improve the sensitivity to meet the requirement for the detection of trace target analytes, a great deal of powerful signal amplification strategies, such as nano material-based assays [51, 52], nuclease-assisted target recycling strategy [53], polymerase-assisted amplification [54] and hybridization-based technology [55], have been proposed and widely applied for nucleic acid assays.
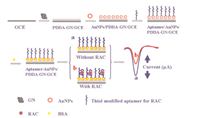
In our group, an ultrasensitive, immobilization-free MEC aptasensor [56] has been developed for the detection of OTA. In this study, we combined the advantages of the discrimination of the aggregation of long and short DNA on a negatively charged ITO electrode, the high selectivity of the aptamer, and the high efficiency of exonuclease-catalyzed target recycling amplification to improve the sensitivity and selectivity of the MEC aptasensor (Fig. 2). Under optimized conditions, the LOD of OTA was 0.004 ng/mL. Subsequently, our group also developed a simple, sensitive, and selective homogeneous MEC biosensor for the detection of melamine [57]. The assay combined the advantages of the high selectivity of the DNA triplex structure containing an abasic site (AP site) to melamine and the high efficiency of exonuclease (Exo) III-assisted recycling amplification. This homogeneous MEC sensor may have a potential prospect in detecting melamine in dairy products and the migration of melamine from multi-category food packaging or application materials.
|
Download:
|
| Fig. 2. Scheme of the immobilization-free electrochemical aptasensor for OTA. Copied with permission [56]. Copyright 2015, American Chemical Society. | |
2.2. Paper-based electrochemical sensor
With the purposes of reducing cost, simplifying instrumentation, and incorporating functionality, paper was applied as a Copied with permission [56]. simple, flexible, and reliable platform for analytical devices [58]. Since the 1960s, paper-based analytical devices (PADs) have been popularized in POC applications [59]. As the most familiar application of PADs, the lateral flow immunoassay has been broadly used for the detection of small molecules and biomarkers [60]. The low cost and ease of remediating filter paper make it a good candidate for POC applications [61]. Paper-based devices have gained increasing attention, especially after high-speed coating and printing techniques were well-established [62].
So far, a number of detection methods have been applied to PAD assays, such as electrochemistry [63], electrochemiluminescence [64], colorimetry [65], Raman [66], fluorescence [67, 68], mass spectrometry [69]. Among them, electrochemical methods may be the most promising one in the POCT application [70] because they are quantitative, have potential to be miniaturized, have low power requirements, and require only simple detection instrumentation. Therefore, we mainly focus on recent advancements of paper-based sensors with electrochemical detection in this review.
To date, many low-cost analytical devices based on paper have been developed and should be useful for applications in public health, environmental monitoring and food safety, especially in the developing world. Nie et al. fabricated paper-based EC sensing devices (PEDs), and the performance of the developed method was evaluated [71]. The PEDs comprised microfluidic channels on paper that were patterned by photolithography or wax printing, and electrodes screen-printed from conducting inks. Delaney et al. described the first approach that combined paper microfluidics with ECL detection [72]. Inkjet printing has also been adopted to produce paper microfluidic substrates, which are combined with screen-printed electrodes (SPEs) to create simple, cheap, dispos-able sensors that can be read without a traditional photo detector. For instance, an amperometric device with addressable electrode arrays was proposed for high throughput, low cost, small consumption, simple operation, and high sensitivity for the combination diagnosis of certain tumors [73].
In order to meet the requirements of low-resource settings and diagnostics at the POCT, Glavan et al. designed an MEC sensor based on ELISA [74]. The device was fabricated entirely in hydrophobic paper, which was produced by the silanization of paper with decyltrichlorosilane. It comprised two zones separated by a central crease: an embossed microwell, of which the antigen or antibody immobilization and recognition events occurred on the surface, and a detection zone where the electrodes were printed. The LOD of the electrochemical sandwich ELISA for Plasmodium falciparum in spiked human serum was 4 ng/mL, and this value was in the range of clinically relevant concentrations. Rattanarat et al. constructed a simple, inexpensive PAD in a three-dimensional (3D) configuration [75]. The readout of detection results could be performed with a dual mode, that being colorimetric and electrochemical. The LOD was as low as 0.25 ng (Cd and Pb). It is believed that the developed device could be accessible for both the developing and developed world.
To further simplify the paper-based analytical devices, a battery-powered MEC-sensing platform was proposed, in which the output was reported using an electrochromic display [76]. The platform was fabricated based on paper fluidics and used a Prussian blue spot electrodeposited on an indium-doped tin oxide thin film as the electrochromic indicator. The integrated metal/air battery powered both the electrochemical sensor and the electro-chromic read-out, which were in electrical contact via a paper reservoir. The sample activated the battery, and the presence of analyte in the sample initiated the color change of the Prussian blue spot. The entire system was assembled on the lab bench, without the need for clean room facilities. The applicability of the device to point-of-care sensing was demonstrated by the qualita-tive detection of 0.1 mmol/L glucose and H2O2 in artificial urine samples. Li et al. showed a simple, paper-folding-based MEC sensor that could detect a 30-base nucleotide sequence characteristic of DNA from the hepatitis B virus (HBV) [77], and the signal amplification was achieved within two stages. First, silver nanoparticle labels provided a maximum amplification factor of 250, 000. Second, magnetic microbeads were concentrated at a detection electrode and provided an additional ~25-fold amplification. Therefore, a lower LOD of 85 pmol/L was achieved, making it a candidate for POC applications.
Smart phones are convenient, suitable for personal detection, and have been applied in the POCT. Wang et al. demonstrated a label-free, smart-phone-based electrochemical white blood cell (WBC)-counting device on micro porous paper with patterned gold microelectrodes [78]. In the presented method, WBCs separated from whole blood were trapped by the paper with microelectrodes (Fig. 3). Therefore, WBCs trapped on the paper led to the ion diffusion blockage on microelectrodes, and cell concentration was determined by peak current on the microelectrodes measured by a differential pulse voltammeter. The quantitative results were collected by a smart phone wirelessly within 1 min. The sample volume was only 10 μL with a high repeatability, as low as 10% in CV (coefficient of variation). The unique smart phone, paper-based MEC sensor ensures fast cell quantification to achieve rapid and low-cost WBC analysis at the POC under challenging conditions.

|
Download:
|
| Fig. 3. Cell counting sensor design and principle. (a) System diagram and electrochemical principle for WBC count. D is diffusion coefficient. i is the electrochemical current. (b) Gold three-electrodes on PVDF (polyvinylidene fluoride) membrane paper. (c) Scanning electron microscopy image of trapped WBC (white blood cell) on PVDF and microporous structure of PVDF membrane. (d) Nyquist plot of electrochemical impedance spectroscopy (EIS) showing the diffusion impedance upward bending by adding more Hela cells on the membrane electrode. The over-potential in EIS is 0.5 V with frequency sweeping from 10 Hz to 100 kHz. (e) Tafel plot of the membrane electrode and wire electrode in 1 mmol/L [Fe(CN)6]3 /[Fe(CN)6]4- and 10 mmol/L PBS at the scan rate of 0.1 mV/s. (f) Simulation of cyclic voltammetry method at different diffusion coefficient in the environment of 1 mmol/L [Fe(CN)6]3 /[Fe(CN)6]4- and the inset is the peak current density change with diffusion coefficient. Copied with permission [78]. Copyright 2016, Elsevier B.V. | |
2.3. Microfluidics-based electrochemical sensor
Microfluidics-based electrochemical sensors provide numerous advantages in clinical diagnostics, environmental monitoring, biomedical research, and food safety. These sensors utilize microfluidic channels to control fluid flow to regions of the chip where a variety of procedures occur, including reagent mixing, affinity-based binding, signal transduction, and even cell culturing [79]. Microfluidic devices require 2–3 orders of magnitude (nanoliters as opposed to microliters) fewer reagents to perform similar assays. Also, these devices can increase the speed at which some biological events occur due to the smaller confinement of the species within the channels [80]. Microfluidics-based EC sensors can be integrated within microfluidic devices using lithography and etching techniques, which can provide label-free detection. In addition, these devices are inexpensive to produce and require little work on the part of the technician to operate [81].
Many microfluidic-based MEC sensors have been developed for POCT application. Uludag et al. described an electrode array and microfluidics-based integrated biochip for the quantitation of the tumor marker prostate specific antigen (PSA) [82]. The LODs were 0.2 ng/L in buffer and 1 ng/L in 75% human serum. This new platform has potential as an automated POC device for clinical use because it is likely to be applicable to numerous other clinical target analytes for which appropriate antibodies are available. Subsequently, they developed a newly integrated and fully automated microfluidic-based MEC sensor device prototype that has potential to be used for cancer biomarker POCT [83]. Especially, this system allowed the formation of a ~7 μL capacity flow cell on the electrode array with the necessary microfluidic and electronic connections with one move of a handle. Samples from a nearby hospital were tested using the developed device. The obtained results were satisfactory when compared with the hospital results, indicating the potential use for POCT. Bravo et al. integrated a bio-affinity nano-platform into a microfluidic immunosensor based on monoclonal bispecific tri-functional antibodies for the electrochemical determination of epithelial cancer biomarker [84]. Regiart et al. developed a microfluidic immunosensor based on a mesoporous silica platform and CMK-3/poly-acrylamide-co-methacrylate of dihydrolipoic-acid-modified gold electrode to realize cancer biomarker detection [85]. For small molecules, Olcer et al. proposed a new microfluidic-based MEC sensing platform to detect mycotoxins. The sensing platform, called real-time electrochemical profiling (REP), relies on real-time electrochemical immunoassay detection [86].
The integration of an MEC sensor on a microfluidic chip provides continuous measurement and collection of electrical signals from targets [87]. An ITO-electrode-based microfluidic MEC sensor was developed for multiplexed quantitative differentiation of bacteria with real-time electrochemical monitoring of loop-mediated isothermal amplification (LAMP) reactions [88]. Micro-fluidic-based MEC DNA sensors, which featured quick responses for target DNA, enabled real-time melting curve analysis when detecting SNPs involved in Alzheimer's disease [89]. A non-biofouling polyethylene glycol (PEG) based microfluidic chip was integrated with functionalized nanoporous alumina membrane for the simultaneous electrochemical detection of two types of bacteria, Escherichia coli O157:H7 and Staphylococcus aureus from the mixed samples [90]. An anion exchange membrane sensor functionalized with a complementary oligo probe was integrated onto a microfluidic device with nucleic acid extraction and pre-concentration units [91]. The microRNA biomarker (miRNA 146a) associated with oral cancer was detected according to the shift in the current-voltage curve with the developed EC sensor.
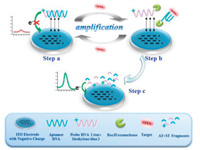
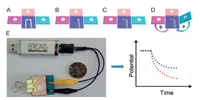
Current paper-based microfluidic EC sensors are commonly planar devices used for the detection of ions. However, these devices have not been designed for the potentiometric biosensing of proteins or small molecule analytes. Arya et al. presented a new biosensor platform using bio receptors modified porous two-dimensional (2D) membrane based off-surface matrix for on-chip electrochemical immunoassay [92]. Differential pulse voltammet-ric studies of anti-TNF-α/FNAB/PC-Au for protein biomarker (TNF-α) detection and estimation in undiluted serum indicated that the immunosensor system can detect TNF-α linearly in the 100 pg/mL to 100 ng/mL range with insignificant interference from other cytokines and serum proteins. 3D printing provided a powerful platform in developing paper-based MEC sensors in the development of POCT devices [93]. The same group proposed a concept of using off-surface matrix modified for capturing biomolecules for on-chip MEC sensing [94]. In this study, a 3D matrix made by laser engraving of polymethyl methacrylate (PMMA) sheet as off-surface matrix was integrated in very close vicinity to the electrode surface. The results for estimating protein biomarker (TNF-α) in undiluted serum using the developed device revealed that the system can detect TNF-α in the 100 pg/mL to 100 ng/mL range with a LOD of 119 nA/(ng/mL), with negligible interference from serum proteins and other cytokines. Qin et al. fabricated a new 3D origami paper-based device for the biosensing of proteins and small molecules in a simple, portable, and cost-effective way (Fig. 4) [95]. A solid-contact ion-selective electrode was integrated with an all-solid-state reference electrode in the 3D origami paper-based device. The device was made by impregnating the paper with appropriate bio receptors and reporting reagents on different zones. Versatile potentiometric bioassays can be performed by folding and unfolding the paper structures. A USB-controlled miniaturized electrochemical detector can be used for simple and in situ measurements. Using butyrylcholinesterase as a model enzyme, the device was then successfully applied for the detection of enzyme activities and organophosphate pesticides involved in the enzymatic system as inhibitors.
|
Download:
|
| Fig. 4. Schematic of the 3D origami potentiometric paper-based device: (A) without the carbon and Ag/AgCl electrodes; (B) with the carbon and Ag/AgCl electrodes; (C) with the ISE and RE membranes; and (D) with the integrated cell for potentiometric detection. (E) The origami paper-based device can be integrated with a miniaturized electrochemical analyzer (USB-controlled) for potentiometric biosensing. Copied with permission [95]. Copyright 2016, Wiley Publishing Group. | |
2.4. Miniaturized capillary electrophoresis-based sensor
The enrichment of targets, separation of multi analytes, and avoiding of background interference are considered as key factors in the analysis of trace amounts of analytes in complex sample matrices. Capillary electrophoresis (CE) provides a powerful platform for the above purpose. Especially, miniaturized CE exhibits promising potential in POC [96]. Moreover, miniaturized CE, such as microchip electrophoresis (ME), represents one of the most well-established examples for how the miniaturization could offer us new analytical possibilities [97].
A number of ME-based EC sensors have been developed for POCT application. Chand et al. performed separation, aliquoting, and detection of amino thiols on an ME-based electrochemical sensor with an inverted double Y-shaped microchannel [98]. Garcia et al. reported the coupling between ME and copper nanowires (CuNWs) for the selective analysis of mono saccharides in honey samples [99]. Petroni et al. developed a simple method based on an ME device with screen-printed-based electrodes for the amperometric detection of nitrite and ascorbate [100]. The lower sensitivity was considered to be the main drawback of the EC sensors, due to the extremely low sample volumes introduced into MEs-based EC sensors, but the required high sensitivity could be achieved by exploiting the surface characteristics of MEs coupled with nanomaterials [101]. Carbon nanostructure materials [102, 103] and metal nanomaterials [104, 105] have been broadly applied to improve the ME-based MEC sensors [106].
With electrophoresis separation, specific signal detection can be selectively retrieved, in which only the signals detected at a particular molecular weight are considered positive regardless of other signals appearing at different positions. Kongsuphol et al. developed a miniaturized electrophoresis electrochemical protein sensor (MEEPS) platform that is capable of detecting specific and multiplexed signals from a small volume of sample (10 μL) as well as reducing the effect of background interferences [107]. The multiplexed capability and the reduced-effect of background interference were achieved using the electrophoresis unit of the MEEPS (Fig. 5). The small footprint system and sensitive signal detection were enabled via an electrochemical sensors unit. Using cytokines as the sample, results obtained from MEEPS revealed a dynamic range of detection of representative cytokine, TNF-α, at 10 ng/mL to 20 μg/mL and demonstrated multiplexed detections of four different cytokines (IL-6, IFN-γ, TNF-α and IL-8) from only 10 μL of sample. Additionally, the platform's small form factor and ease of use also highlighted its potential to be deployed as a POC device for the detection of multiple biomarkers. To improve POC quantification using miniaturized CE-based sensor, the chip-to-chip variabilities inherent in disposable, single-use devices must be addressed. Bidulock et al. proposed a novel method of integrating an internal standard (ISTD) into the microchip by adding it to the background electrolyte (BGE) instead of the sample, thus eliminating the need for the additional sample manipulation, microchip redesigns, and/or system expansions required for traditional ISTD usage [108].

|
Download:
|
| Fig. 5. Miniaturized electrophoresis-electrochemical protein sensor (MEEPS) for multiplexed protein detections. (A) Scheme illustrates the design of the biosensor comprisingtwo operating units-the electrophoresis separation unit (left) and the electrochemical sensor unit (right). (B) Picture of real PMMA electrophoresis separation unit. (C) Picture of the CSGM chip employed for electrochemical sensor. Inset shows a scanning electron microscope (SEM) image of the sensing area of the CSGM chip which reveals the comb-structured feature. Copied with permission [107]. Copyright 2017, Elsevier B.V. | |
2.5. Screen electrode-based microchip
Since the 1990s, screen-printed techniques have been applied to fabricate EC sensors with high-volume production at low cost and high reproducibility and reliability [109]. Screen-printed techniques, as one of the cutting-edge technologies, make EC sensors more convenient for various analytical purposes [110].
In particular, it provides a precise control over the dimensions of screen-printed electrodes (SPEs), excellent uniformity, and the potential for miniaturization [111]. Therefore, the use of screen-printing technology in the serial production of disposable and low-cost electrodes for the electrochemical determination of a wide range of substances is currently undergoing widespread growth. Especially, the SPEs have been designed for miniaturization of electrochemical analytical systems. These disposable sensors can be easily modified in various ways and are also suitable for measuring multiple biological samples, as only a small sample volume is required [112]. SPE technology has drawn tremendous attention, and its market size is estimated to reach $300 billion in the future [113].
However, the selection of the different materials as inks of SPEs is very important according to the specific purpose of the EC sensor [114]. Carbon materials or modifications of carbon materials, such as graphene [115, 116], graphite [117, 118], fullerene [119] and carbon nanotubes (CNTs) [120], are the most commonly used materials for screen-printed inks. The nanomaterials have engen-dered great impact on EC sensors [121], especially for SPE-based EC sensors in POCT application. These materials are used to enhance the immobilization efficiency of biological molecules and acceler-ate the charge transfer rate on the electrode surface. Furthermore, they can increase the electrochemical mediation to amplify signals from SPEs [122, 123]. However, other materials, such as gold nanomaterials [124, 125] and platinum [126], have also been reported for the construction of the working electrodes in several SPEs-based sensors.
SPE-based MEC sensors, in particular, play a pivotal role in healthcare [127] and security domains [128]. However, the majority of these SPE high-performance devices are mechanically fragile, which limit their full realization of minimization. Wang et al. presented the first example of an all-printed, inexpensive, highly stretchable, CNT-based EC sensor (Fig. 6) [129]. The important novel and attractive aspect of the developed method is the judicious preparation of a highly stretchable CNT-based ink and its combination with a judiciously designed pattern that provides the sensor with two degrees of stretchability to accommodate extreme strains of up to 500%.

|
Download:
|
| Fig. 6. (A) Image of the stencil employed for printing the stress-enduring stretchable devices. Schematics showing (B) large-scale printed stretchable device arrays along with their various applications (inset shows an image of a printed CNT-based array device) and (C) the two degrees of stretching design-induced (1 st stretching) and intrinsic (2nd stretching) stretchability, enabling the printed arrays to accommodate high levels of strains along with parameters defining the curvature of a free-standing serpentine interconnect (top right). Photographs of stretchable array under (D) 0% and (E) 175% linear, (F) 180 torsional, and (G) 5 mm indention strains. Scale bar for images D–G =1 cm. Copied with permission [129]. Copyright 2016, American Chemical Society. | |
Ionic liquids (ILs) have attracted more attention regarding the improvement of SPEs sensors because they are highly thermally and chemically stable, have a negligible vapor pressure, tunable viscosity, electrolytic conductivity, have a wide electrochemical window, and have good extractability for organic compounds and metal ions [130]. In 2013, a miniaturized, solid-state "forensic finger" was used to detect the explosive 2, 4-dinitrotoluene from gunshot residue [124]. The sensor was fabricated by screen-printing the electrode onto a flexible substrate, followed by the printing of an ionic liquid with a water-soluble diacrylate polymer. Chaiyo et al. developed a screen printed MEC sensor modified with a Nafion/IL/graphene composite for the simultaneous detection of zinc, cadmium, and lead [131]. A disposable SPE sensor modified by IL n-octylpyridinum hexafluorophosphate (OPFP) and graphene (GR) was fabricated and used for the sensitive detection of Cd(II) in soil [132]. A systematical study of the prolonged amperometric detection of oxygen in ILs was achieved by the use of mechanical polishing to activate platinum screen-printed electrodes (Pt-SPEs) [133]. The results demonstrat-ed that the lowest LOD (of ca. 0.1 vol% O2) was found on polished SPEs using long-term chronoamperometry, with the most stable responses observed in N-butyl-N-methyl-pyrrolidinium bis(tri-fluoromethylsulfonyl)imide [C4mpyrr][NTf2]. The characteristics of ILs combined SPE technology to fabricate high performance MEC sensors exhibiting a promising potential for POCT application.
It is well-known that multi-analyte approaches provide more information for each single sample and lead to faster and lower-cost assays. SPE-based sensors provide a powerful platform. Shi et al. reported an ultrasensitive EC immunoassay for the rapid detection of inter leukin-6 (IL-6) and matrix metallopeptidase-9 (MMP-9) [134]. The method utilized PS@PDA-metal nano compo-sites based on graphene nano ribbon (GNR)-modified heated SPCE. A sandwich strategy was adopted to achieve the simultaneous detection of MMP-9 and IL-6 based on a heated SPCE sensor in the range of 10 5-103 ng/mL with LOD of 5 fg/mL and 0.1 pg/mL, respectively. Most importantly, satisfactory results were also obtained in the practical samples. Subsequently, Sánchez-Tirado et al. developed a dual SPE sensor modified with 4-carboxyphenyl-functionalized double-walled carbon nanotubes for the simulta-neous determination of the cytokines IL-1β and TNF-α (Fig. 7) [135]. Under the optimized conditions that affect the immuno-sensor performance, the dual immunosensor allowed linear ranges extending 0.5–100 pg/mL and 1–200 pg/mL for IL-1β and TNF-α, respectively. These improvements of linear ranges were adequate for the determination of the cytokines in clinical samples, and lower LODs were achieved at 0.38 pg/mL (IL-1β) and 0.85 pg/mL (TNF-α). In 2015, an attractive study was reported by McKenzie et al. [136], wherein they described the development of a novel multi analyte SPE MEC sensor for the detection of analytes central to cellular bioenergetics: glucose, lactate, oxygen, and pH.
|
Download:
|
| Fig. 7. Schematic display of the different steps involved in the preparation of the dual electrochemical immunosensor for multiplexed determination of IL-1β and TNF-α cytokines. Copied with permission [135]. Copyright 2017, Elsevier B.V. | |
2.6. Other kinds of miniaturized electrochemical systems
Efforts have been conducted to develop new tools to detect biomarkers for infectious diseases and cancers [137, 138]. The requirements for integrated, user-friendly, MEC-based POCT devices for the detection of target analytes in resource-limited settings should be rapid, sensitive, and quantitative. Some novel and MEC sensors have been developed to meet these requirements. Yu et al. developed a miniaturized, disposable electrochemical sensor for the detection of 2, 4, 6-trinitrotoluene with an ionic liquid gel-polymer electrolyte film [139]. Ribet et al. presented an ultra-miniaturized EC biosensor for continuous glucose monitoring (CGM) [140]. The aim of this work was to demonstrate the possibility of an overall reduction in sensor size to allow minimally invasive glucose monitoring in the interstitial fluid in the dermal region, in contrast to larger state-of-the-art systems, which are necessarily placed in the subcutaneous layer. Pursey et al. designed a multi-sensor microchip array that efficiently detected three specific bladder cancer DNA markers simultaneously with a LOD of 250 fmol/L, well below the amount of DNA markers found in urine samples [141]. Yuan et al. reported an EC sensor combining two distinguishable magnetic nanoprobes (DNA1/Fe3O4 NPs/Thi and DNA2/Fe3O4 NPs/Fc) with a target-triggered hybridization chain reaction (HCR) strategy for the simultaneous detection of micro-RNA-141 (miR-141) and microRNA-21 (miR-21) [142]. Hu et al. established a novel, label-free, electrochemical strategy based on the unique electro-catalytic activity of graphene oxide (GO)-supported terminal deoxynucleotidyl transferase (TdT)-generated C-rich DNA nano tail templated silver nanoclusters [143]. Suher-man et al. described an ultrasensitive method for Hg2+ detection by undertaking linear sweep voltammetry with a silver-nanoparticle-modified glassy carbon electrode in aqueous solutions containing Hg2+ [144]. Especially, Yang et al. designed a novel intelligent microscale electrochemical device with an integrated reagent delivery system [145]. The successful adoption of a bubble-based cartridge to the SPE system led to an automatic and rapid sample delivery at the electrode surface in one step with minimal user intervention. This low-cost and portable device has overcome the major barrier for quantitative POCTs with electrochemical detection.
Cell-based electrochemical biosensors have emerged in the last decade as a sensitive and noninvasive technique [146]. An in situ electrochemical method was developed to integrate cell culture pretreatment with detection in the same cell culture dish [147]. The electrochemical behaviors of human breast cancer (MCF-7) and human cervical carcinoma (HeLa) cells based on graphene- or carbon-nanotubes-modified glass carbon electrode were investi-gated [148]. However, due to the limitations of the size and sensitivity of the electrodes, this method was only used for the detection of cells cultured in 60-mm or 100-mm dishes with high amounts of cells. Zhu et al. presented a sensitive, cell-based MEC biosensor to assess the toxicity of priority pollutants in an aquatic environment [149]. On the basis of the hybrid-composite-modified pencil graphite electrode, the cell culture and detection vessel were miniaturized to a 96-well plate instead of the traditional culture dish to improve the portability in POCT.
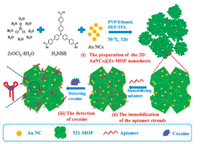
Nanostructured metal-organic frameworks (nMOFs) materials have attracted increasing attention as well due to their broad potential applications in the fields of sensors for detecting various targets [150]. Moreover, some excellent characteristics such as π-π stacking, hydrogen bonding and electrostatic force, can be formed between special functional groups on the organic linkers of nMOFs and nucleic acid sequences with negatively charges [151]. A series of MEC aptasensors combined with MOFs have been developed to detect targets [152]. Liu et al. proposed a tunable MEC aptasensor based on analogous Zr-MOFs for the detection of protein, which exhibited high sensitivity, satisfactory repeatability, and applica-bility in real samples [153]. Subsequently, the same group prepared 2D Zr-MOFs nanosheets embedded with Au nanoclusters (denoted as 2D AuNCs@521-MOF) via a one-pot method [154]. The optimized 2D AuNCs@521-MOF nanosheets exhibited strong bioaffinity toward biomolecule-bearing phosphate groups. On the basis of 2D AuNCs@521-MOF nanosheets, they constructed an EC aptasensor, which can be used to successfully detect cocaine through the specific binding interactions between cocaine and aptamer strands (Fig. 8).
|
Download:
|
| Fig. 8. Schematic of the fabrication of electrochemical biosensing based on AuNCs@Zr-MOF-based banosheets for detection of cocaine, including (i) preparation of the 2D AuNCs@Zr-MOF nanosheets, (ii) immobilization of the aptamer strands, and (iii) detection of cocaine. Copied with permission [154]. Copyright 2017, American Chemical Society. | |
3. Point-of-care applications
A typical POCT assay is affordable, specific, sensitive, portable, rapid, and user- friendly, which also makes it suitable for use in low-resource settings [155]. Various analytical methods based on MEC sensors could meet the typical requirement of POCT, which exhibit great potential and promising perspective in POCT application.
Microfluidic-based MEC sensors are currently popular in POCT application, mainly due to the lower sample consumption for multiplex analysis on a single system [156]. Narang et al. proposed a novel, microfluidic-paper-based MEC sensor for the detection of ketamine [157]. Their study involved the fabrication of anano-hybrid-based electrochemical micro-fluidic paper-based analytical device with a low LOD of 0.001 nmol/L/mL that could be achieved for ketamine. Fan et al. proposed a wireless POCT system for the detection of neuron-specific enolase (NSE) [158]. The wireless POCT system was developed as a microfluidic paper-based MEC sensor, and a low LOD of 10 pg/mL was achieved. The accuracy of a POCT whole blood glucose sensor can be considerably influenced by hematocrit (Hct). Therefore, Weng et al. developed an on-chip Hct correction protocol, incorporated to meet the clinical demands [159].
Paper-based MEC sensors have exhibited promising potential and great prospective in POCT application. Fujimoto et al. presented a paper-based MEC glucose sensor with a high performance of measurement ranging from 0 to 10 mmol/L [160]. Boonyasit et al. developed a selective, novel, three-dimensional, paper-based electrochemical impedance device (3D-PEID) for the multiplexed determination of diabetes markers using a single EIS platform [161]. The results indicated that the 3D-PEID not only provided a precise measurement with a wide linear concentration range but also offered a great sensitivity for the total haemoglobin and HbA1c values within the clinically relevant ranges. Hu et al. developed a paper-based EC sensor that allowed simple, rapid identification and quantification of various chemicals from microliter-size samples with the aid of a handheld multi-meter [162]. Ge et al. established a low-cost, simple, portable, and sensitive paper-based EC sensor for the detection of K-562 cells in POCT [163]. Their group developed a series of paper-based EC sensors for the sensitive detection of small molecules [164], proteins [165], and DNA [166]. Most recently, Cao et al. developed an MEC immunofiltration analysis combining paper-based analyt-ical devices for the detection of human chorionic gonadotropin (HCG) with a low LOD of 0.36 mIU/mL [167]. The proposed MEC sensor was used to test HCG in real human serum and obtained satisfactory results. Li et al. developed a pen-on-paper strategy based on two custom-made pens, including a wax pen and a conductive-ink pen, to detect glucose in POCT [168].
SPE-based MEC sensors have been broadly applied to POCT applications. Piermarini et al. fabricated an uricase biosensor based on an SPE modified with Prussian blue for the detection of uric acid in human blood serum with a lower LOD [169]. Cinti et al. fabricated an MEC sensor based on inkjet-printed Prussian blue nanoparticle-modified SPE to realize the sensitive detection of cholesterol [170]. With the gold nanoparticle modified screen-printed carbon electrode, Labib et al. developed a three-mode MEC sensor for quantitative detection of ultra-low levels of miRNAs [171]. The sensor facilitated three detection modalities based on hybridization (H-SENS), p19 protein binding (P-SENS), and protein displacement (D-SENS). The combined three-mode sensor (HPD-SENS) identified as low as 5 amol/L or 90 molecules of miRNA per 30 μL of sample without PCR amplification, and it also operated within the dynamic range from 10 amol/L to 1 μmol/L. Azzouzi et al. proposed a novel MEC sensor based on gold nanoparticles anchored on reduced graphene oxide for the sensitive detection of l-lactate tumor biomarker [172].
In addition, Park et al. reported an MEC-based protease sensor for the detection of botulinum neurotoxin type E light chain (BoNT/ E-LC) [173]. The LODs for BoNT/E-LC in phosphate-buffered saline were 0.1 ng/mL for an incubation period of 15 min and 5 fg/mL for an incubation period of 4 h. The LOD in commercial bottled water was 1 ng/mL for an incubation period of 15 min. The developed sensor was selective to BoNT/E-LC among the four types of BoNTs tested. It was claimed that the MEC based protease sensor meets the requirements for POCT. ECL is now established as an important, highly sensitive detection strategy for the development of POCT devices [174]. Yan et al. developed an ECL immunosensor for the highly sensitive determination of carcinoembryonic antigen in human serum sample [175]. The developed method possessed a low LOD of 0.3 pg/mL and would provide a new platform for low-cost, sensitive, specific, multiplex assay and POC diagnosis in public health, environmental monitoring, and the developing world. Hrdý et al. presented a portable lock-in amplifier-based electrochemical sensing system [176]. The basic unit (cluster) consisted of four MEC cells, with each cell containing one pseudo reference electrode (PRE) and one working electrode. All four MECs cells were simultaneously interrogated at different frequencies, with square wave pulses superposed on a saw tooth signal for cyclic voltammetry (CV). This system was built in a portable palm-size format suitable for POC applications and can perform either individual or multiple measurements of active compounds, such as biomarkers.
Streptococcus pneumoniae (SP) is a pathogenic bacterium and a major cause of community-acquired pneumonia that could be fatal if left untreated. Therefore, the rapid and sensitive detection of SP is crucial to enable targeted treatment during SP infections. To meet the requirement, a DNA tetrahedron (DNA TH) with a hollow structure and anchored on gold electrodes was proposed to construct an EC immunosensor for the rapid detection of pneumococcal surface protein A (PspA) peptide and SP lysate from synthetic and actual human samples [177]. This DNA nanostructure-based immunosensor displayed excellent electro-chemical activity toward PspA with a sensitive linear region from 0 to 8 ng/mL of PspA peptide and a low LOD of 0.218 ng/mL.
4. Conclusions and perspectivesIn this review, we have summarized the state-of-the-art advancements on various types of MEC sensors and their applications in POCT (Table 1). Especially, a number of innovative MEC-based POCT assays have been presented in both academic research and commercial products. Although great achievement has been highlighted in applications of miniaturized MEC sensors in POCT, there are still challenges to overcome and the need for further improvement.
|
|
Table 1 Summary of miniaturized electrochemical sensors. |
Acknowledgments
The authors thank the China National Key R & D Program (No. 2017YFC1601604) and National Natural Science Foundation of China (NSFC) (No. 21777189) for financially supporting this research. We also thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
| [1] |
H.R.S. Lima, J.S. da Silva, E.A. de Oliveira Farias, et al., Biosens. Bioelectron. 108 (2018) 27-37. DOI:10.1016/j.bios.2018.02.034 |
| [2] |
A. Jang, Z. Zou, K.K. Lee, C.H. Ahn, P.L. Bishop, Talanta 83 (2010) 1-8. DOI:10.1016/j.talanta.2010.07.061 |
| [3] |
N. Hamada, Y. Hashi, S. Yamaki, et al., Chin. Chem. Lett. 30 (2019) 99-102. DOI:10.1016/j.cclet.2018.10.029 |
| [4] |
D.A. McPartlin, R.J. O'Kennedy, Expert Rev. Mol. Diagn. 14 (2014) 979-998. DOI:10.1586/14737159.2014.960516 |
| [5] |
L.J. Zhao, F.Q. Zhao, B.Z. Zeng, Biosen. Bioelectron. 62 (2014) 19-24. DOI:10.1016/j.bios.2014.06.022 |
| [6] |
W.J. Lian, S. Liu, J.H. Yu, et al., Biosens. Bioelectron. 38 (2012) 163-169. DOI:10.1016/j.bios.2012.05.017 |
| [7] |
T. Wang, D. Zhao, X. Guo, et al., Anal. Chem. 86 (2014) 4354-4361. DOI:10.1021/ac500163f |
| [8] |
L. Wei, Y. Lei, H. Fu, J. Yao, ACS Appl. Mater. Interfaces 4 (2012) 1594-1600. DOI:10.1021/am201769u |
| [9] |
D.W. Kimmel, G. LeBlanc, M.E. Meschievitz, D.E. Cliffel, Anal. Chem. 84 (2012) 685-707. DOI:10.1021/ac202878q |
| [10] |
R. Sharma, K.V. Ragavan, M.S. Thakur, K.S.M.S. Raghavarao, Biosens. Bioelectron. 74 (2015) 612-627. DOI:10.1016/j.bios.2015.07.017 |
| [11] |
A.E. Radi, Int. J. Electrochem. Sci. 2011 (2011) 863196. |
| [12] |
Z. Wu, N. Xu, W. Li, J.M. Lin, Chin. Chem. Lett. 30 (2019) 95-98. DOI:10.1016/j.cclet.2018.01.048 |
| [13] |
X. Lin, Y. Wang, M. Zou, T. Lan, Y. Ni, Chin. Chem. Lett. 30 (2019) 1157-1160. DOI:10.1016/j.cclet.2019.04.009 |
| [14] |
M. Li, S. Mao, S. Wang, H.F. Li, J.M. Lin, Sci. China Chem. 62 (2019) 142-150. DOI:10.1007/s11426-018-9327-7 |
| [15] |
L. Zhang, W. Yang, Y. Yang, H. Liu, Z. Gu, Analyst 140 (2015) 7399-7406. DOI:10.1039/C5AN01664A |
| [16] |
S. Tombelli, M. Minunni, M. Mascini, Biosens. Bioelectron. 20 (2005) 2424-2434. DOI:10.1016/j.bios.2004.11.006 |
| [17] |
A.D. Ellington, J.W. Szostak, Nature 346 (1990) 818-822. DOI:10.1038/346818a0 |
| [18] |
Y. Du, S. Dong, Anal. Chem. 89 (2017) 189-215. DOI:10.1021/acs.analchem.6b04190 |
| [19] |
C.I.L. Justino, T.A. Rocha-Santos, A.C. Duarte, Trends Anal. Chem. 29 (2010) 1172-1183. DOI:10.1016/j.trac.2010.07.008 |
| [20] |
K. Metfies, S. Huljic, M. Lange, L.K. Medlin, Biosens. Bioelectron. 20 (2005) 1349-1357. DOI:10.1016/j.bios.2004.05.011 |
| [21] |
S.W. Yeung, T.M.H. Lee, H. Cai, I.M. Hsing, Nucleic Acids Res. 34(2006) e118.
|
| [22] |
Z. Gao, A. Agarwal, A.D. Trigg, et al., Anal. Chem. 79 (2007) 3291-3297. DOI:10.1021/ac061808q |
| [23] |
A.L. Ghindilis, M.W. Smith, K.R. Schwarzkopf, et al., Biosen. Bioelectron. 22 (2007) 1853-1860. DOI:10.1016/j.bios.2006.06.024 |
| [24] |
J.M. Yang, B.T. Dou, R. Yuan, Y. Xiang, Anal. Chem. 88 (2016) 8218-8223. DOI:10.1021/acs.analchem.6b02035 |
| [25] |
A.B. Hashkavayi, J.B. Raoof, Biosens. Bioelectron. 91 (2017) 650-657. DOI:10.1016/j.bios.2017.01.025 |
| [26] |
W. Hou, Z. Shi, Y. Guo, X. Sun, X. Wang, Bioproc. Biosyst. Eng. 40 (2017) 1419-1425. DOI:10.1007/s00449-017-1799-6 |
| [27] |
A. Nezami, R. Nosrati, B. Golichenari, et al., Trends Anal. Chem. 94 (2017) 95-105. DOI:10.1016/j.trac.2017.07.003 |
| [28] |
R. Rapini, G. Marrazza, Bioelectrochemistry 118 (2017) 47-61. DOI:10.1016/j.bioelechem.2017.07.004 |
| [29] |
M. Hasanzadeh, N. Shadjou, M. de la Guardia, Trends Anal. Chem. 89 (2017) 119-132. DOI:10.1016/j.trac.2017.02.003 |
| [30] |
F. Yang, P. Wang, R. Wang, et al., Biosen. Bioelectron. 77 (2016) 347-352. DOI:10.1016/j.bios.2015.09.050 |
| [31] |
A. Bogomolova, E. Komarova, K. Reber, et al., Anal. Chem. 81 (2009) 3944-3949. DOI:10.1021/ac9002358 |
| [32] |
C. Zhu, G. Yang, H. Li, D. Du, Y. Lin, Anal. Chem. 87 (2015) 230-249. DOI:10.1021/ac5039863 |
| [33] |
L. Rivas, C.C. Mayorga-Martinez, D. Quesada-González, et al., Anal. Chem. 87 (2015) 5167-5172. DOI:10.1021/acs.analchem.5b00890 |
| [34] |
Z. Izadi, M. Sheikh-Zeinoddin, A.A. Ensafi, S. Soleimanian-Zad, Biosens. Bioelectron. 80 (2016) 582-589. DOI:10.1016/j.bios.2016.02.032 |
| [35] |
M.A. Tabrizi, M. Shamsipur, L. Farzin, Biosens. Bioelectron. 74 (2015) 764-769. DOI:10.1016/j.bios.2015.07.032 |
| [36] |
Y. Du, B.J. Lim, B. Li, et al., Anal. Chem. 86 (2014) 8010-8016. DOI:10.1021/ac5025254 |
| [37] |
H. Li, N. Arroyo-Curras, D. Kang, F. Ricci, K.W. Plaxco, J. Am. Chem. Soc. 138 (2016) 15809-15812. DOI:10.1021/jacs.6b08671 |
| [38] |
T. Yuan, Z.Y. Liu, L.Z. Hu, G.B. Xu, Chin. J. Anal. Chem. 39 (2011) 972-977. DOI:10.1016/S1872-2040(10)60451-3 |
| [39] |
L. Li, Y. Chen, J.J. Zhu, Anal. Chem. 89 (2017) 358-371. DOI:10.1021/acs.analchem.6b04675 |
| [40] |
M.L. Yang, B.Y. Jiang, J.Q. Xie, et al., Talanta 125 (2014) 45-50. DOI:10.1016/j.talanta.2014.02.061 |
| [41] |
Y. Chen, M.L. Yang, Y. Xiang, R. Yuan, Y.Q. Chai, Nanoscale 6 (2014) 1099-1104. DOI:10.1039/C3NR05499C |
| [42] |
L. Yang, Y. Zhang, R. Li, et al., Biosen. Bioelectron. 70 (2015) 268-274. DOI:10.1016/j.bios.2015.03.067 |
| [43] |
G. Jin, C. Wang, L. Yang, et al., Biosen. Bioelectron. 63 (2015) 166-171. DOI:10.1016/j.bios.2014.07.033 |
| [44] |
Y. Hu, X. Xu, Q. Liu, et al., Anal. Chem. 86 (2014) 8785-8790. DOI:10.1021/ac502008k |
| [45] |
F. Xuan, X. Luo, I.M. Hsing, Anal. Chem. 84 (2012) 5216-5220. DOI:10.1021/ac301033w |
| [46] |
S. Liu, Y. Wang, C. Zhang, Y. Lin, F. Li, Chem. Commun. 49 (2013) 2335-2337. DOI:10.1039/c3cc39082a |
| [47] |
S. Liu, Y. Lin, L. Wang, et al., Anal. Chem. 86 (2014) 4008-4015. DOI:10.1021/ac500426b |
| [48] |
X. Fu, Z. Liu, S. Cai, et al., Chin. Chem. Lett. 27 (2016) 920-926. DOI:10.1016/j.cclet.2016.04.014 |
| [49] |
H. Xu, R. Ye, S. Yang, R. Li, X. Yang, Chin. Chem. Lett. 25 (2014) 29-34. DOI:10.1016/j.cclet.2013.10.011 |
| [50] |
F. Xuan, T.W. Fan, I.M. Hsing, ACS Nano 9 (2015) 5027-5033. DOI:10.1021/nn507282f |
| [51] |
B. Esteban-Fernández de Ávila, E. Araque, S. Campuzano, et al., Anal. Chem. 87 (2015) 2290-2298. DOI:10.1021/ac504032d |
| [52] |
F. Xiao, L. Wang, H. Duan, Biotechnol. Adv. 34 (2016) 234-249. DOI:10.1016/j.biotechadv.2016.01.006 |
| [53] |
X. Zhang, D. Wu, Z. Liu, et al., Chem. Commun. 50 (2014) 12375-12377. DOI:10.1039/C4CC05541A |
| [54] |
X. Zhu, X. Zhou, D. Xing, Biosens. Bioelectron. 31 (2012) 463-468. DOI:10.1016/j.bios.2011.11.016 |
| [55] |
Y. Chen, J. Xu, J. Su, et al., Anal. Chem. 84 (2012) 7750-7755. DOI:10.1021/ac3012285 |
| [56] |
Y. Tan, X. Wei, Y. Zhang, et al., Anal. Chem. 87 (2015) 11826-11831. DOI:10.1021/acs.analchem.5b03314 |
| [57] |
C. Fu, C. Liu, Y. Li, et al., Anal. Chem. 88 (2016) 10176-10182. DOI:10.1021/acs.analchem.6b02753 |
| [58] |
S.K. Mahadeva, K. Walus, B. Stoeber, ACS Appl. Mater. Interfaces 7 (2015) 8345-8362. DOI:10.1021/acsami.5b00373 |
| [59] |
L.A. Bastian, K. Nanda, V. Hasselblad, D.L. Simel, Arch. Fam. Med. 7 (1998) 465-469. DOI:10.1001/archfami.7.5.465 |
| [60] |
G.A. Posthuma-Trumpie, J. Korf, A. van Amerongen, Anal. Bioanal. Chem. 393 (2009) 569-582. DOI:10.1007/s00216-008-2287-2 |
| [61] |
J. Hu, S. Wang, L. Wang, et al., Biosens. Bioelectron. 54 (2014) 585-597. DOI:10.1016/j.bios.2013.10.075 |
| [62] |
A.W. Martinez, S.T. Phillips, E. Carrilho, et al., Anal.Chem. 80 (2008) 3699-3707. DOI:10.1021/ac800112r |
| [63] |
J.C. Cunningham, N.J. Brenes, R.M. Crooks, Anal. Chem. 86 (2014) 6166-6170. DOI:10.1021/ac501438y |
| [64] |
X. Zhang, J. Li, C. Chen, et al., Chem. Commun. 49 (2013) 3866-3868. DOI:10.1039/c3cc40905h |
| [65] |
D.M. Cate, W. Dungchai, J.C. Cunningham, J. Volckens, C.S. Henry, Lab Chip 13 (2013) 2397-2404. DOI:10.1039/c3lc50072a |
| [66] |
B. Li, W. Zhang, L. Chen, B. Lin, Electrophoresis 34 (2013) 2162-2168. DOI:10.1002/elps.201300138 |
| [67] |
K. Scida, B.L. Li, A.D. Ellington, R.M. Crooks, Anal. Chem. 85 (2013) 9713-9720. DOI:10.1021/ac402118a |
| [68] |
L. Luo, X. Li, R.M. Crooks, Anal. Chem. 86 (2014) 12390-12397. DOI:10.1021/ac503976c |
| [69] |
J. Ho, M.K. Tan, D.B. Go, et al., Anal. Chem. 83 (2011) 3260-3266. DOI:10.1021/ac200380q |
| [70] |
B. Liu, D. Du, X. Hua, X. Yu, Y. Lin, Electroanalysis 26 (2014) 1214-1223. DOI:10.1002/elan.201400036 |
| [71] |
Z. Nie, C.A. Nijhuis, J. Gong, et al., Lab Chip 10 (2010) 477-483. DOI:10.1039/B917150A |
| [72] |
J.L. Delaney, C.F. Hogan, J. Tian, W. Shen, Anal. Chem. 83 (2011) 1300-1306. DOI:10.1021/ac102392t |
| [73] |
S. Ge, L. Ge, M. Yan, et al., Chem. Commun. 48 (2012) 9397-9399. DOI:10.1039/c2cc34887j |
| [74] |
A.C. Glavan, D.C. Christodouleas, B. Mosadegh, et al., Anal. Chem. 86 (2014) 11999-12007. DOI:10.1021/ac5020782 |
| [75] |
P. Rattanarat, W. Dungchai, D. Cate, et al., Anal. Chem. 86 (2014) 3555-3562. DOI:10.1021/ac5000224 |
| [76] |
H. Liu, R.M. Crooks, Anal. Chem. 84 (2012) 2528-2532. DOI:10.1021/ac203457h |
| [77] |
X. Li, K. Scida, R.M. Crooks, Anal. Chem. 87 (2015) 9009-9015. DOI:10.1021/acs.analchem.5b02210 |
| [78] |
X. Wang, G. Lin, G. Cui, X. Zhou, G.L. Liu, Biosen.Bioelectron. 90 (2017) 549-557. DOI:10.1016/j.bios.2016.10.017 |
| [79] |
H. Ben-Yoav, P.H. Dykstra, T. Gordonov, W.E. Bentley, R. Ghodssi, J. Vis. Exp. 91 (2014) e51797.
|
| [80] |
H. Song, R.F. Ismagilov, J. Am. Chem. Soc. 125 (2003) 14613-14619. DOI:10.1021/ja0354566 |
| [81] |
H. Craighead, Nature 442 (2006) 387-393. DOI:10.1038/nature05061 |
| [82] |
Y. Uludag, G. Kokturk, Microchim. Acta 182 (2015) 1685-1691. DOI:10.1007/s00604-015-1477-9 |
| [83] |
Y. Uludag, F. Narter, E. Saglam, etal., et al., Anal.Bioanal.Chem. 408 (2016) 7775-7783. DOI:10.1007/s00216-016-9879-z |
| [84] |
K. Bravo, F.G. Ortega, G.A. Messina, et al., Clin. Chim. Acta 464 (2017) 64-71. DOI:10.1016/j.cca.2016.11.012 |
| [85] |
M. Regiart, M.A. Fernández-Baldo, Villarroel-Rocha J., et al., Anal. Chim. Acta 963 (2017) 83-92. DOI:10.1016/j.aca.2017.01.029 |
| [86] |
Z. Olcer, E. Esen, T. Muhammad, et al., Biosen. Bioelectron. 62 (2014) 163-169. DOI:10.1016/j.bios.2014.06.025 |
| [87] |
L. Zhang, B. Ding, Q. Chen, et al., Trends Anal. Chem. 94 (2017) 106-116. DOI:10.1016/j.trac.2017.07.013 |
| [88] |
J. Luo, X.E. Fang, D.X. Ye, et al., Biosens. Bioelectron. 60 (2014) 84-91. DOI:10.1016/j.bios.2014.03.073 |
| [89] |
A.H.J. Yang, K. Hsieh, A.S. Patterson, et al., Angew. Chem. Int. Ed. 53 (2014) 3163-3167. DOI:10.1002/anie.201310059 |
| [90] |
F. Tian, J. Lyu, J. Shi, F. Tan, M. Yang, Sens.ActuatorsB-Chem. 225 (2016) 312-318. DOI:10.1016/j.snb.2015.11.059 |
| [91] |
Z. Slouka, S. Senapati, S. Shah, et al., Talanta 145 (2015) 35-42. DOI:10.1016/j.talanta.2015.04.083 |
| [92] |
S.K. Arya, P. Kongsuphol, M.K. Park, Biosen. Bioelectron. 91 (2017) 721-727. DOI:10.1016/j.bios.2017.01.033 |
| [93] |
H.N. Chan, M.J.A. Tan, H. Wu, Lab Chip 17 (2017) 2713-2739. DOI:10.1039/C7LC00397H |
| [94] |
S.K. Arya, P. Kongsuphol, M.K. Park, Biosen. Bioelectron. 92 (2017) 542-548. DOI:10.1016/j.bios.2016.10.063 |
| [95] |
J. Ding, B. Li, L. Chen, W. Qin, Angew. Chem. Int. Ed. 55 (2016) 13033-13037. DOI:10.1002/anie.201606268 |
| [96] |
J. Koenka, J. Saiz, P. Rempel, P.C. Hauser, Anal. Chem. 88 (2016) 3761-3767. DOI:10.1021/acs.analchem.5b04666 |
| [97] |
A. Martin, D. Vilela, A. Escarpa, Electrophoresis 33 (2012) 2212-2227. DOI:10.1002/elps.201200049 |
| [98] |
R. Chand, S.K. Jha, K. Islam, et al., Biosen. Bioelectron. 40 (2013) 362-367. DOI:10.1016/j.bios.2012.08.009 |
| [99] |
M. Garcia, A. Escarpa, Electrophoresis 35 (2014) 425-432. DOI:10.1002/elps.201300458 |
| [100] |
J.M. Petroni, B.G. Lucca, V.S. Ferreira, Anal. Chim. Acta 954 (2017) 88-96. DOI:10.1016/j.aca.2016.12.027 |
| [101] |
L. García-Carmona, A. Martín, T. Sierra, M.C. González, A. Escarpa, Electrophoresis 38 (2017) 80-94. DOI:10.1002/elps.201600232 |
| [102] |
W. Harreither, R. Trouillon, P. Poulin, et al., Anal. Chem. 85 (2013) 7447-7453. DOI:10.1021/ac401399s |
| [103] |
D. Vilela, A. Martin, M.C. González, A. Escarpa, Analyst 139 (2014) 2342-2347. DOI:10.1039/C4AN00025K |
| [104] |
P. Liang, M. Sun, P. He, L. Zhang, G. Chen, Food Chem. 190 (2016) 64-70. DOI:10.1016/j.foodchem.2015.05.059 |
| [105] |
Y. Wang, H. Xu, J. Luo, et al., Biosens. Bioelectron. 83 (2016) 319-326. DOI:10.1016/j.bios.2016.04.062 |
| [106] |
X. Jia, S. Dong, E. Wang, Biosen. Bioelectron. 76 (2016) 80-90. DOI:10.1016/j.bios.2015.05.037 |
| [107] |
P. Kongsuphol, G.C.F. Lee, S.K. Arya, S.Y. Chiam, M.K. Park, Sens. Actuators BChem. 244 (2017) 823-830. DOI:10.1016/j.snb.2017.01.026 |
| [108] |
A.C.E. Bidulock, P. Dubský, A.T. van den Berg, J.C.T. Eijkel, Anal. Chem. 89 (2017) 2886-2892. DOI:10.1021/acs.analchem.6b04172 |
| [109] |
G. Hughes, K. Westmacott, K.C. Honeychurch, et al., Biosensors 6 (2016) 50.
|
| [110] |
F. Arduini, S. Cinti, V. Scognamiglio, D. Moscone, G. Palleschi, Anal. Chim. Acta 959 (2017) 15-42. DOI:10.1016/j.aca.2016.12.035 |
| [111] |
M. Tudorache, C. Bala, Anal. Bioanal. Chem. 388 (2007) 565-578. DOI:10.1007/s00216-007-1293-0 |
| [112] |
A.C. Power, A. Morrin, Electroanalytical sensor technology, in: M.A.A. Khalid (Ed.), Electrochemistry, InTech, Rijeka, 2013, pp. 141-178.
|
| [113] |
A. Kamyshny, S. Magdassi, Small 10 (2014) 3515-3535. DOI:10.1002/smll.201303000 |
| [114] |
R.A.S. Couto, J.L.F.C. Lima, M.B. Quinaz, Talanta 146 (2016) 801-814. DOI:10.1016/j.talanta.2015.06.011 |
| [115] |
D. Zang, M. Yan, S. Ge, L. Ge, J. Yu, Analyst 138 (2013) 2704-2711. DOI:10.1039/c3an00109a |
| [116] |
M. Cui, S. Liu, W. Lian, et al., Analyst 138 (2013) 5949-5955. DOI:10.1039/c3an01190a |
| [117] |
A. Avramescu, S. Andreescu, T. Noguer, et al., Anal. Bioanal. Chem. 374 (2002) 25-32. DOI:10.1007/s00216-002-1312-0 |
| [118] |
E. Fernández, L. Vidal, J. Iniesta, et al., Anal.Bioanal.Chem. 406 (2014) 2197-2204. DOI:10.1007/s00216-013-7415-y |
| [119] |
A. Chen, S. Chatterjee, Chem. Soc. Rev. 42 (2013) 5425-5438. DOI:10.1039/c3cs35518g |
| [120] |
L. Agüí, P. Yáñez-Sedeño, J.M. Pingarrón, Anal. Chim. Acta 622 (2008) 11-47. DOI:10.1016/j.aca.2008.05.070 |
| [121] |
L. Syedmoradi, M. Daneshpour, M. Alvandipour, et al., Biosen. Bioelectron. 87 (2017) 373-387. DOI:10.1016/j.bios.2016.08.084 |
| [122] |
Z. Cui, F.R. Poblete, G. Cheng, et al., J. Mater. Res. 30 (2015) 79-85. DOI:10.1557/jmr.2014.347 |
| [123] |
M.U. Ahmed, M.M. Hossain, M. Safavieh, et al., Crit. Rev. Biotechnol. 36 (2016) 495-505. |
| [124] |
M. Liu, J. Xiang, J. Zhou, H. Ding, J. Electroanal. Chem. 640 (2010) 1-7. DOI:10.1016/j.jelechem.2009.12.020 |
| [125] |
A. Jirasirichote, E. Punrat, A. Suea-Ngam, O. Chailapakul, S. Chuanuwatanakul, Talanta 175 (2017) 331-337. DOI:10.1016/j.talanta.2017.07.050 |
| [126] |
W. Wei, X. Zong, X. Wang, et al., Food Chem. 135 (2012) 888-892. DOI:10.1016/j.foodchem.2012.06.037 |
| [127] |
E. Carrilho, A.W. Martinez, G.M. Whitesides, Anal. Chem. 81 (2009) 7091-7095. DOI:10.1021/ac901071p |
| [128] |
A.J. Bandodkar, A.M. O'Mahony, et al., Analyst 138 (2013) 5288-5295. DOI:10.1039/c3an01179h |
| [129] |
A.J. Bandodkar, I. Jeerapan, J.M. You, R. Nunez-Flores, J. Wang. Nano Lett. 16 (2016) 721-727. DOI:10.1021/acs.nanolett.5b04549 |
| [130] |
P. Sun, D.W. Armstrong, Anal. Chim. Acta 661 (2010) 1-16. DOI:10.1016/j.aca.2009.12.007 |
| [131] |
S. Chaiyo, E. Mehmeti, K. Žagar, et al., Anal. Chim. Acta 918 (2016) 26-34. DOI:10.1016/j.aca.2016.03.026 |
| [132] |
G. Zhao, Y. Si, H. Wang, G. Liu, Int. J. Electrochem. Sci. 11 (2016) 54-64. |
| [133] |
J. Lee, D.W.M. Arrigan, D.S. Silvester, Anal. Chem. 88 (2016) 5104-5111. DOI:10.1021/acs.analchem.5b04782 |
| [134] |
J.J. Shi, T.T. He, F. Jiang, Abdel-Halim E.S., J.J. Zhu, Biosen. Bioelectron. 55 (2014) 51-56. DOI:10.1016/j.bios.2013.11.056 |
| [135] |
E. Sánchez-Tirado, C. Salvo, A. González-Cortés, et al., Anal. Chimi. Acta 959 (2017) 66-73. DOI:10.1016/j.aca.2016.12.034 |
| [136] |
J.R. McKenzie, A.C. Cognata, A.N. Davis, J.P. Wikswo, D.E. Cliffel. Anal. Chem. 87 (2015) 7857-7864. |
| [137] |
B. Lam, J. Das, R.D. Holmes, et al., Nat. Commun. 4 (2013) 2001.
|
| [138] |
Y. Sameenoi, K. Koehler, J. Shapiro, et al., J. Am. Chem. Soc. 134 (2012) 10562-10568. DOI:10.1021/ja3031104 |
| [139] |
H.A. Yu, J. Lee, S.W. Lewis, D.S. Silvester, Anal. Chem. 89 (2017) 4729-4736. DOI:10.1021/acs.analchem.7b00679 |
| [140] |
F. Ribet, G. Stemme, N. Roxhed, Biosen. Bioelectron. 90 (2017) 577-583. DOI:10.1016/j.bios.2016.10.007 |
| [141] |
J.P. Pursey, C. Yu, E. Stulz, K.P. Mi, P. Kongsuphol, Sens. Actuators B-Chem. 251 (2017) 34-39. DOI:10.1016/j.snb.2017.05.006 |
| [142] |
Y.H. Yuan, Y.D. Wu, B.Z. Chi, et al., Biosen. Bioelectron. 97 (2017) 325-331. DOI:10.1016/j.bios.2017.06.022 |
| [143] |
Y. Hu, Q. Zhang, Z. Guo, et al., Biosen. Bioelectro. 98 (2017) 91-99. DOI:10.1016/j.bios.2017.06.017 |
| [144] |
A.L. Suherman, K. Ngamchuea, E.E.L. Tanner, et al., Anal. Chem. 89 (2017) 7166-7173. DOI:10.1021/acs.analchem.7b01304 |
| [145] |
F. Yang, X. Zuo, Z. Li, et al., Adv. Mater. 26 (2014) 4671-4676. DOI:10.1002/adma.201400451 |
| [146] |
H. Ben-Yoav, R.O. Almog, Y. Sverdlov, et al., Electrochim. Acta 82 (2012) 109-114. DOI:10.1016/j.electacta.2012.03.042 |
| [147] |
H. Qin, Q. Gao, H. Niu, et al., Analyst 138 (2013) 3372-3375. DOI:10.1039/c3an00379e |
| [148] |
J. Li, J. Song, S. Bi, et al., J. Hazard. Mater. 313 (2016) 238-243. DOI:10.1016/j.jhazmat.2015.09.031 |
| [149] |
X. Zhu, G. Wu, N. Lu, X. Yuan, B. Li, J. Hazard. Mater. 324 (2017) 272-280. DOI:10.1016/j.jhazmat.2016.10.057 |
| [150] |
Y. Li, S. Zhang, D. Song, Angew. Chem. Int. Ed. 52 (2013) 710-713. DOI:10.1002/anie.201207610 |
| [151] |
P. Ramaswamy, N.E. Wong, G.K.H. Shimizu, Chem. Soc. Rev. 43 (2014) 5913-5932. DOI:10.1039/C4CS00093E |
| [152] |
M. Chen, N. Gan, Y. Zhou, et al., Sens. Actuators B-Chem. 242 (2017) 1201-1209. DOI:10.1016/j.snb.2016.08.185 |
| [153] |
C.S. Liu, Z.H. Zhang, M. Chen, et al., Chem. Commun. 53 (2017) 3941-3944. DOI:10.1039/C7CC00029D |
| [154] |
F. Su, S. Zhang, H. Ji, et al., ACS Sens. 2 (2017) 998-1005. DOI:10.1021/acssensors.7b00268 |
| [155] |
Y. Song, Y.Y. Huang, X. Liu, et al., Trends Biotechnol. 32 (2014) 132-139. DOI:10.1016/j.tibtech.2014.01.003 |
| [156] |
H. Ashiba, M. Fujimaki, K. Awazu, T. Tanaka, M. Makishima, Sens. Biosensing Res. 7 (2016) 121-126. DOI:10.1016/j.sbsr.2016.01.012 |
| [157] |
J. Narang, N. Malhotra, C. Singhal, et al., Biosen. Bioelectron. 88 (2017) 249-257. DOI:10.1016/j.bios.2016.08.043 |
| [158] |
Y. Fan, J. Liu, Y. Wang, J. Luo, et al., Biosens. Bioelectron. 95 (2017) 60-66. DOI:10.1016/j.bios.2017.04.003 |
| [159] |
C.W. Weng, T.J. Cheng, R.L.C. Chen, B.C. Hsieh, Int. J. Electrochem. Sci. 12 (2017) 4990-4999. |
| [160] |
T. Fujimoto, S. Kawahara, Y. Fuchigami, et al., Int. J. Electr. Comput. Eng. 7 (2017) 1423-1429. |
| [161] |
Y. Boonyasit, O. Chailapakul, W. Laiwattanapaisal, Anal. Chim. Acta 936 (2016) 1-11. DOI:10.1016/j.aca.2016.05.047 |
| [162] |
J. Hu, C.H.T. Yew, X. Chen, et al., Talanta 165 (2017) 419-428. DOI:10.1016/j.talanta.2016.12.086 |
| [163] |
S. Ge, L. Zhang, Y. Zhang, et al., Talanta 145 (2015) 12-19. DOI:10.1016/j.talanta.2015.05.008 |
| [164] |
F. Liu, S.G. Ge, J.H. Yu, M. Yan, X.R. Song, Chem. Commun. 50 (2014) 10315-10318. DOI:10.1039/C4CC04199B |
| [165] |
S.G. Ge, L. Ge, M. Yan, et al., Chem. Commun. 48 (2012) 9397-9399. DOI:10.1039/c2cc34887j |
| [166] |
J.J. Lu, S.G. Ge, L. Ge, M. Yan, J.H. Yu, Electrochim. Acta 80 (2012) 334-341. DOI:10.1016/j.electacta.2012.07.024 |
| [167] |
L.L. Cao, C. Fang, R.S. Zeng, et al., Biosens. Bioelectron. 92 (2017) 87-94. DOI:10.1016/j.bios.2017.02.002 |
| [168] |
Z.D. Li, F. Li, Y. Xing, et al., Biosens. Bioelectron. 98 (2017) 478-485. DOI:10.1016/j.bios.2017.06.061 |
| [169] |
S. Piermarini, D. Migliorelli, G. Volpe, et al., Sens. Actuators B-Chem. 179 (2013) 170-174. DOI:10.1016/j.snb.2012.10.090 |
| [170] |
S. Cinti, F. Arduini, D. Moscone, et al., Sens. Actuators B-Chem. 221 (2015) 187-190. DOI:10.1016/j.snb.2015.06.054 |
| [171] |
M. Labib, N. Khan, S.M. Ghobadloo, et al., J. Am. Chem. Soc. 135 (2013) 3027-3038. DOI:10.1021/ja308216z |
| [172] |
S. Azzouzi, L. Rotariu, A.M. Benito, et al., Biosens. Bioelectron. 69 (2015) 280-286. DOI:10.1016/j.bios.2015.03.012 |
| [173] |
S. Park, Y.M. Shin, J. Seo, J.J. Song, H. Yang, Analyst 141 (2016) 2481-2486. DOI:10.1039/C6AN00251J |
| [174] |
W. Gao, M. Saqib, L. Qi, W. Zhang, G. Xu, Curr. Opin. Electrochem. 3 (2017) 4-10. DOI:10.1016/j.coelec.2017.03.003 |
| [175] |
J. Yan, M. Yan, L. Ge, S. Ge, J. Yu, Sens. Actuators B-Chem. 193 (2014) 247-254. DOI:10.1016/j.snb.2013.11.107 |
| [176] |
R. Hrdý, H. Kynclová, I. Klepácová, M. Barto 9šík, P. Neužil, Anal. Chem. 89 (2017) 8731-8737. DOI:10.1021/acs.analchem.7b00776 |
| [177] |
J. Wang, M.C. Leong, E.Z.W. Leong, W.S. Kuan, D.T. Leong, Anal. Chem. 89 (2017) 6900-6906. DOI:10.1021/acs.analchem.7b01508 |
 2020, Vol. 31
2020, Vol. 31