b School of Automotive Engineering, Dalian University of Technology, Dalian 116024, China;
c Department of Gastroenterology, Affiliated Zhongshan Hospital of Dalian University, Dalian 116001, China;
d Department of Radiology, Second Affiliated Hospital of Dalian Medical University, Dalian 116027, China
Polylactide (PLA) is one of the most attractive polymers due to its good biodegradability, biocompatibility, renewability and excellent processability. As a result, it is widely used in biomedical applications such as tissue engineering and drug delivery system [1]. However, PLA cannot be detected by X-ray due to its low electron density of atomic composition such as C, H and O, which limited its further development in tracing technology and interventional therapy field. Therefore, endowing PLA with inherent radiopacity is of significance to X-ray imaging in clinical applications. A conventional method of X-ray visibility was to incorporate inorganic metal ions (such as zirconium dioxide or barium salts) [2] or iodine-based contrast agents (such as triiodobenzoic acid and iohexol) into polymers. However, these heavy metal ions spontaneously leak from matrix and induce systematic toxicity. To overcome the limitation, much attention has been paid for the formation of stable combination of contrast agents and polymers. It is worth noting that the covalent bonding via chemical synthesis is a promising manner, which can provide a stable and long-term X-ray imaging and avoid possible leakage of contrast agents [3-6].
Because PLA lacks a reactive site for introduction of iodinated groups, it is a challenging work to directly covalent bonding of iodine-containing compounds onto PLA backbones [7]. Recently, we reported a facile method of end-group modification to prepare X-ray opaque PLA using triiodobenzoic acid (TIBA) as end-capping agent [8].However, the incorporation of iodine content is limited due to the less bonding site. A new strategy of in-chain functionalization through the combination of ring opening copolymerization of functional comonomer and post-polymerization of oxime "Click" reaction [9], is developed to obtain higher iodine content of PLA materials. The object of this study is to design and synthesize high iodine content PLA copolymers with intrinsic radiopacity, and evaluate the capacity of X-ray imaging by Micro-CT.
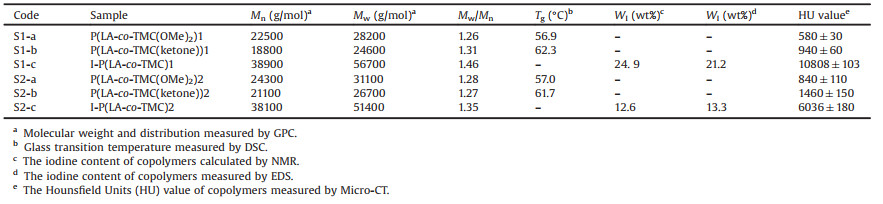
As shown in Fig. 1, a functional cyclic carbonate was prepared and used as comonomer to copolymerize with L-lactide followed by a postpolymerization deprotection step towards ketonecontaining PLA copolymers. An advance prepared iodinated hydroxylamine was then imparted into the target polymers through oxime "Click" postpolymerization. As a consequence, a new radiopaque iodinated PLA material was achieved.
|
Download:
|
| Fig. 1. Synthetic route of iodinated PLA copolymer. | |
The functional comonomer and iodination molecule were synthesized according to the previous reported procedure [9, 10]. Detailed experimental procedures are available in Supporting information. All the prepared small molecules and polymers were characterized using proton nuclear magnetic resonance (1H NMR) in Figs. S1 and S2 (Supporting information) and Fig. 1. The data were listed here:
Ⅱ: 1H NMR (400 MHz, CDCl3): δ 2.35 (1H, OH), 3, 7 (2H, CH2), 3.3 (3H, CH3).
Ⅲ: 1H NMR (400 MHz, CDCl3): δ 4.4 (2H, CH2), 3.35 (3H, CH3).
Ⅳ: 1H NMR (400 MHz, CDCl3): δ 1.45 (3H, LA = CH3), 4.12 (2H, TMC = CH2), 3.18 (3H, TMC = CH3), 5.14 (H, LA = CH).
Ⅴ: 1H NMR (400 MHz, CDCl3): δ 1.45 (3H, LA = CH3), 4.83 (2H, TMC = CH2), 5.14 (H, LA = CH).
Ⅶ: 1H NMR (400 MHz, CDCl3): δ 7.92 (m, 1H, Ar = H), 7.90 (1H, phthalimido), 7.78 (1H, phthalimido), 7.61 (1H, Ar = H), 7.48 (1H, Ar = H), 7.01 (1H, Ar = H), 5.35 (2H, C2H4ICH2-).
Ⅷ: 1H NMR (400 MHz, CDCl3): δ 7.81 (1H, Ar=H), 7.35–7.49 (2H, Ar = H), 7.0 (1H, Ar = H), 6.51 (2H, -ONH2), 4.69 (2H, C6H4ICH2-).
Ⅸ: 1H NMR (400 MHz, CDCl3, δ): 1.45 (3H, LA = CH3), 4.83 (2H, TMC = CH2), 5.08 (2H, C6H4ICH2-), 5.14 (H, LA = CH), 6.9 (1H, Ar = H), 7.2 (1H, Ar = H), 7.5 (1H, Ar = H), 7.9 (1H, Ar = H).
The chemical structures of these copolymers during the synthetic processes were traced and confirmed by 1H NMR. As shown in Fig. 2, the peak c of OCH3 in TMC unit at 3.18 ppm completely disappeared after deprotection, and the peak b of OCH2 at 4.12 ppm shifted to the peak at 4.83 ppm (b') after deprotection, indicating the ketal group has been changed into the ketone group. As a result, it can be concluded that the ketone-containing PLA copolymer has been successfully prepared. Furthermore, the appearance of new peaks e + f+g + h (6.9–7.2 ppm, 7.5–7.9 ppm) originated from the protons of phenyl groups in O-(2-iodobenzyl) hydroxylamine confirmed that the iodinated hydroxylamine has been imparted into the target polymers by oxime "Click" reaction. Therefore, 1H NMR spectra demonstrate the successful synthesis of iodinated PLA copolymers.

|
Download:
|
| Fig. 2. 1HNMR spectra of pristine, deprotection and iodinated copolymers. | |
The molecular weight and thermal properties of these copolymers were monitored by GPC and DSC, respectively. And the data were listed in Table 1. As shown in Fig. S3 (Supporting information), single and symmetric eluting curves could be observed for all the copolymers. The molecular weight of copolymers showed a little reduction after deprotection and then showed a considerable increment after oxime "Click" reaction. The differences can be explained by a little chain scission during deprotection process and the increment of hydrodynamic volume of the molecular chains by anchoring a large volume of contrast agent, respectively. During thermal analysis, all the copolymers did not show crystallization and melting peak, and showed a slight increment of glass transition temperature after deprotection.
|
|
Table 1 Data on molecular weight and X-ray radiopacity of the resulting copolymers. |
The radiopacity intensity directly depends on the content of iodine within the copolymers. Thus, the iodine contents were calculated by both 1H NMR (Fig. 2) and EDS (Fig. 3), listed in Table 1. The iodine contents could be easily regulated by the feedstock. X-ray imaging of I-P(LA-co-TMC) materials is obtained by using X-ray radiography and Micro-CT. All these samples were made into strips with 2 mm thickness, and the X-ray images were obtained by X-radiographic examination under standard X-ray condition. As shown in Fig. 3a, the four non-iodinated samples exhibited similar low radiopacity, where as the other two iodinated samples exhibited high radiopacity. Further, Fig. 3b showed the in vivo radiopacity of a little of iodinated materials implanted into rabbit, which both exhibited good radiopacity and X-ray identification due to the high contents of iodine atoms. The excellent X-ray visibility indicated a great potential application as interventional device or implant.

|
Download:
|
| Fig. 3. (a) Micro-CT images of cylinder-shaped samples; (b) In vivo X-ray images of S1-c and S2-c cylinder-shaped samples; (c) EDS image of S1-c; (d) EDS image of S2-c. | |
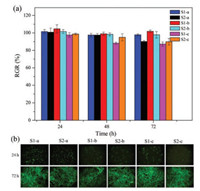
To evaluate the cell cytotoxicty of these new synthesized copolymers, in vitro biological properties as cytotoxicity against 3T3-Swiss albinowas tested via direct contact method using Alamar blue. The cell viability on tissue culture plate was set as control. The cell viability was measured on a microplate reader (Varioskan FLash, Thermo Fisher) at intervals of 24 h. As shown in Fig. 4a, all the PLA copolymers showed high values of the relative growth rate (RGR%) above 75%, demonstrating non-cytotoxicity of synthesized polymers. Furthermore, the cell morphology after incubation for 24 h and 72 h were shown under fluorescent microscope in Fig. 4b. It can be seen that the cells proliferated rapidly and spread out on the surface regardless of the samples with or without iodine atoms.
|
Download:
|
| Fig. 4. (a) Cell relative growth rate (RGR%) culture on the synthesized materials incubated for 24, 48 and 72 h. (b) Fluorescence images of cell proliferation incubated for 24 h and 72 h. | |
In conclusion, biodegradable radiopaque iodinated PLA copolymers have been successfully synthesized by in-chain functionalization through the combination of ring opening copolymerization and oxime "Click" reaction. Two different iodine contents copolymers were carefully characterized to being definite molecular structure, good X-ray visibility, and low cell cytotoxicty. It is a feasible method to develop biodegradable biomaterials with inherent radiopacity.
AcknowledgmentsThe work was financially supported by the National Natural Science Foundation of China (No. 31500767), the Natural Science Foundation of Liaoning Province of China (No. 20180510037), and the Fundamental Research Funds for the Central Universities (No. DUT19LAB27).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.062.
| [1] |
Z.W. You, Y.D. Wang, Adv. Funct. Mater. 22 (2012) 2812-2820. DOI:10.1002/adfm.201102024 |
| [2] |
H. Lusic, M.W. Grinstaff, Chem. Rev. 113 (2013) 1641-1666. DOI:10.1021/cr200358s |
| [3] |
T.R. Olsen, L.L. Davis, S.E. Nicolau, et al., Acta Biomater. 20 (2015) 94-103. DOI:10.1016/j.actbio.2015.03.021 |
| [4] |
B. Nottelet, J. Coudane, M. Vert, Biomaterials 27 (2006) 4948-4954. DOI:10.1016/j.biomaterials.2006.05.032 |
| [5] |
Q. Ma, K. Lei, J. Ding, et al., Polym. Chem. 8 (2017) 6665-6674. DOI:10.1039/C7PY01411B |
| [6] |
Y. Zou, Y. Wei, G. Wang, et al., Adv. Mater. 29 (2017) 1603997. DOI:10.1002/adma.201603997 |
| [7] |
B. Nottelet, V. Darcos, J. Coudane, Eur. J. Pharm. Biopharm. 97 (2015) 350-370. DOI:10.1016/j.ejpb.2015.06.023 |
| [8] |
W.H. Wang, Z.Y. Wei, L. Sang, et al., Eur. Polym. J. 108 (2018) 337-347. DOI:10.1016/j.eurpolymj.2018.09.018 |
| [9] |
S.E. Nicolau, L.L. Davis, C.C. Duncan, et al., J. Polym. Sci. Part A: Polym. Chem. 53 (2015) 2421-2430. DOI:10.1002/pola.27706 |
| [10] |
J.R. Weiser, P.N. Zawaneh, D. Putnam, Biomacromolecules 12 (2012) 977-986. |
 2020, Vol. 31
2020, Vol. 31