Polymer capsules have attracted increasing attention due to their potential applications, especially in the area of drug delivery. Over the past two decades, layer-by-layer (LbL) assembly has been one of the mostly used methods for the fabrication of polymer capsules, which involves sequential adsorption of functional polymers onto sacrificial templates followed by the removal of the templates [1-4]. LbL method is a universal method for the fabrication of polymer capsules due to its applicability to numerous templates and building materials. Importantly, the shape and size of capsules can be retained after the removal of the sacrificial templates [5, 6]. Polymer capsule shape as well as surface chemistry and size has been recognized as prominent properties for the influence of bio-nano interactions, such as cellular uptake and vascular dynamics [7-10]. For example, cell uptake of polymer capsules with hemispherical or spherical shapes are significantly different [11]. Drug-loaded spherical capsules had more cytotoxic than the corresponding cubes within the first 10 h of cell incubation [12]. Therefore, an appropriate template is the key factor to design LbL capsules with different morphologies for the investigation of bio-nano interactions.
A range of templates, such as Au nanoparticles [13], polystyrene particles [14] and silica particles [15-17] have been used as templates for the assembly of LbL capsules. However, these common templates have been typically removed under harsh condition, such as organic solvents, oxidizing agents, or toxic solution, which could influence the morphology and nanostructure of capsules. Although metal carbonates (e.g., CaCO3, CdCO3 and MnCO3 microparticles) have attracted considerable interests due to the mild condition (e.g., EDTA) for the removal of the templates [18-21], the metal carbonate particles used as templates for LbL capsules are spherical microparticles [22, 23]. Therefore, it is highly desirable to develop alternative templates with tunable shapes that can be dissolved under mild condition.
Recently, cuprous oxide (Cu2O) nanocrystals with different shapes were achieved by simply adjusting the synthetic conditions (e.g., chemical ratios, temperature and stirring speed) [24, 25]. For example, the short-range-ordered Cu2O mesoporous spheres with uniform pores were synthesized using water-insoluble cuprous chloride (CuCl) as the intermediate to slow down the hydrolysiscondensation rates of Cu+ ions [26]. A series of Cu2O nanocrystals (~200 nm) with systematic shape evolution, from cubic to faceraised cubic, edge-and corner-truncated octahedral, all-cornertruncated rhombic dodecahedral, {100}-truncated rhombic dodecahedral, and rhombic dodecahedral structures, have been reported by the adjustment of the dosage of hydroxylamine hydrochloride (NH2OH·HCl) at room temperature [27]. A variety of Cu2O particles architectures, evolved from cubes through truncated cubes, cubooctahedrons, truncated octahedrons and finally to octahedrons, can be obtained by simply adjusting the amount of poly(N-vinylpyrrolidone) (PVPON) [28]. In addition, Cu2O nanocrystals can be easily dissolved at weak acid pH or in sodium thiosulfate (Na2S2O3) solution. Therefore, Cu2O particles as sacrificial templates for LbL capsules could offer some distinct advantages: (1) Cu2O particles are inexpensive and easy to synthesize and can be dissolved under mild condition; (2) A series of Cu2O nanocrystals with tunable shapes can be achieved via changing the synthetic condition.
Herein, we report the LbL assembly of tannic acid (TA)-based (Molecular structures of TA see Fig. S1 in Supporting information) polymer capsules using Cu2O particles as templates with different shapes and investigate the influence of the capsule shapes on cell association. Cu2O particles with cubic, tetradecahedral or spherical shapes were synthesized by hydrothermal method. TA, bovine serum (BSA), PVPON and poly(ethylene glycol) (PEG) were used as building blocks for the LbL assembly of capsules with multilayers of (PVPON/TA/BSA/TA/BSA/PEG) using Cu2O particles as templates, which can be removed with Na2S2O3 solution (Scheme 1). The polymer capsules with an average size of 1 μm were used for the investigation of the influence of capsule shape on cell association. As a result, capsules with cubic geometry have the highest cell association compared to tetradecahedral and spherical capsules.

|
Download:
|
| Scheme 1. Schematic illustration of LbL assembly of (PVPON/TA/BSA/TA/BSA/PEG) capsules using Cu2O templates with cubic, tetradecahedral and spherical shapes, respectively. | |
Monodisperse cubic, tetradecahedral Cu2O particles were synthesized via a hydrothermal process as described in previous work [28]. Spherical Cu2O particles were prepared under high reaction concentration by so-called "rapid nucleation slow growth" model. Scanning electron microscopy (SEM) (Figs. 1a-c) and transmission electron microscopy (TEM) (Figs. 1d-f) were used to confirm the shapes of the prepared Cu2O particles. As shown in Fig. 1, monodisperse cubic, tetradecahedral and spherical Cu2O particles were successfully synthesized. Based on the dynamic light scattering measurements, the size of cubic, tetradecahedral and spherical Cu2O particles is similar (~1 μm, Fig. 2a), which is consistent with the results of SEM and TEM measurements. SEM (Figs. 1a and b) and TEM (Figs. 1d and e) images distinctly demonstrated that the cubic and tetradecahedral Cu2O particles geometry were formed, which was achieved by adjusting the amount of added PVPON. It can be deduced that PVPON had acted as a capping agent and that preferential adsorbed on the {111} planes of the Cu2O particles. The adsorption kinetics of the surface activities on {111} could control the volume ratio of {111} over {100}, which resulted in the tetradecahedral Cu2O particles.

|
Download:
|
| Fig. 1. SEM (a-c) and TEM (d-f) images of Cu2O particles with cubic (a, d), tetradecahedral (b, e) and spherical (c, f) shapes. | |

|
Download:
|
| Fig. 2. . Size distribution (a) and XRD patterns (b) of Cu2O particles with cubic, tetradecahedral and spherical shapes. | |
To further confirm the fabricated Cu2O particles, X-ray diffraction (XRD) experiments were performed (Fig. 2b). XRD patterns of these crystals corresponded well to the standard diffraction pattern of cuprite Cu2O. All the diffraction peaks can be indexed to cuprite Cu2O in spite of the deviation of the relative diffraction intensity. As shown in SEM image (Fig. 1c), spherical Cu2O particles have a rougher surface than cubic and tetradecahedral Cu2O particles, which indicates that the crystallization of spherical Cu2O particles is not perfect. The result was consistent with the XRD results.
The degradation of Cu2O particles in the presence of Na2S2O3 solution was investigated. After adding 1 mol/L Na2S2O3 aqueous solutions into the Cu2O particles suspensions, it became transparent solution from cloudy suspension after 1 h incubation (Fig. 3). Na2S2O3 solution with low concentration can also dissolve the Cu2O particles but it required longer time. Cu2O particles can be decomposed in the presence of Na2S2O3, where Na2S2O3 was used as a coordinating etchant to form a soluble [Cu2(S2O3)x]2-2x complex [29]. It indicates that Cu2O particles are potentially good candidates to serve as a sacrificial template for LbL capsules.

|
Download:
|
| Fig. 3. Photographs of Cu2O aqueous suspensions before (a, b and c is cube, tetradecahedron and sphere, respectively) and after (a', b' and c' is cube, tetradecahedron and sphere, respectively) adding 1 mol/L of Na2S2O3 aqueous solutions. | |
TA which is a natural antioxidant containing active phenolic hydroxyl groups has been widely used as hydrogen bond donor. BSA as a natural protein has high content of reactive functional groups (e.g., amine and carboxyl groups) and good biocompatibility, which is beneficial to the realization of capsule functionalization. Herein, TA and BSA were selected building blocks for capsule assembly. Firstly, PVPON and TA were sequentially adsorbed on Cu2O particles by forming hydrogen-bonded. BSA was then assembled on the TA layers. As a flexible hydrophilic polymer, 8-arm-PEG succinimidyl succinate (8-arm-PEG-NHS) was modified on BSA layers via forming the amide bonds. Cu2O particles with cubic, tetradecahedral, and spherical shapes were used as sacrificial templates, for the assembly of (PVPON/TA/BSA/TA/BSA/PEG) multi-layers. After the removal of Cu2O templates with Na2S2O3 solution, different morphology capsules were obtained.
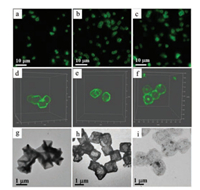
Fluorescence microscopy, confocal laser scanning microscopy (CLSM), and TEM were used to investigate the structures of the capsules. To visualize the capsules under fluorescence microscopy, BSA was labeled with Fluorescein isothiocyanate (FITC) for capsule assembly. As observed in fluorescence microscopy images, despite different surface morphologies and shapes of the templates, monodisperse cubic (Fig. 4a), tetradecahedral (Fig. 4b) and spherical (Fig. 4c) capsules templated Cu2O particles were successfully obtained. In addition, the capsules coated with 8-arm-PEG-NHS can enhance the dispersity of capsules compared with the capsules without coating of 8-arm-PEG-NHS (Fig. S2 in Supporting information). 3D reconstruction of CLSM images demonstrated the morphologies of capsules with different shapes (Figs. 4d-f), where cubic and tetradecahedral capsules replicated the multiple flat facets of the respective templates. TEM images (Figs. 4g-i) showed that the templates were totally decomposed leading to a polymeric replica of the templates. The morphology of the monodisperse capsules was with robust walls after air-dry.
|
Download:
|
| Fig. 4. Fluorescence microscopy images (a-c), 3D reconstruction of CLSM images (d-f) and TEM images (g-i) of cubic (a, d, g), tetradecahedral (b, e, h) and spherical (c, f, i) (PVPON/TA/BSA/TA/BSA/PEG) capsules. Green fluorescence was from the FITClabeled BSA. | |
Cytotoxicity of capsules as a drug delivery vehicle is a critical factor for therapeutic delivery applications. Herein, HeLa cells were used to check the cytotoxicity of the capsules. HeLa cells were incubated with capsules at various capsule-to-cell ratios of 10:1, 20:1, 50:1 and 100:1 for 24 h. Fig. 5a shows the cell viability as a function of capsule concentration. The result showed that the cytotoxicity of the capsules is almost negligible, even at a capsuleto-cell ratio of 100:1, regardless of capsule shapes (i.e., cube, tetradecahedron or sphere). The low toxicity of the capsules enables them to be good candidates for drug delivery.

|
Download:
|
| Fig. 5. (a) Cytotoxicity of capsules with different shapes after 24 h incubation in complete media supplied with 10% FBS against HeLa cells. Cell viability was evaluated by the MTT assay (mean ± SD, n = 3). (b) Cell association of cubic, tetradecahedral and spherical (PVPON/TA/BSA/TA/BSA/PEG) capsules with HeLa cells after 4 h incubation. | |
To assess cell interactions of capsules, HeLa cells were incubated with cubic, tetradecahedral, and spherical capsules at a capsule-to-cell ratio of 10:1, 20:1, 50:1 and 100:1, respectively. Cell association of the capsules with different shapes was investigated after 4 h incubation using flow cytometry. Cell association of cubic, tetradecahedral and spherical capsules all increased along with the increasing capsule-to-cell ratio (Fig. 5b). Importantly, cell association of cubic capsules was significantly higher compared to spherical and tetradecahedral capsules. Specifically, cell association with cubic capsules was about 4-fold and 9-fold higher compared to tetradecahedral and spherical capsules, respectively, when the capsule-to-cell ratio was 50:1. When increasing the capsule-to-cell ratio to 100:1, cell association with cubic capsules was approximately 2-fold or 9.5-fold of tetradecahedral or spherical capsules, respectively. Cell association of capsules with different shapes was further confirmed by CLSM. The FITC-labeled capsules were incubated with cells at a capsuleto-cell ratio of 100:1 for 4 h. As shown in Fig. S3 (Supporting information), cubic capsules resulted in the highest cell association compared with tetradecahedral and spherical capsules. Less than 20% of HeLa cells interacted with spherical capsules. These results are consistent with that of flow cytometry.
The differences of cell association of capsules with different shapes could be due to the contact area and adhesion energy gain. Herein, the cell membrane in comparison to capsules is approximatively considered as a plane and the maximum contact area is caculated for cubic and tetradecahedral capsules (Table S1 in Supporting information). Compared with tetradecahedral capsules, cubic capsules have larger contact area with cells, while spherical capsuels have the smallest contact area (Fig. S4 in Supporting information). Higher contact area usually increase the chance for cell association. In addition, the modeling studies of the lipid membrane binding indicate that capsule attachment is also controlled by adhesion energy gain of the capsule–cell membrane contact and energy cost of the cell membrane deformation [30, 31]. Less membrane deformation energy leads to higher adhesion between capsules and cells. Previous work has proved that cubic particles have less membrane deformation energy and thus higher adhesion [32, 33]. Therefore, cubic capsules with a higher contact surface area and a higher adhesion have the highest cell association among the capsules with three different shapes. Similar results have been reported by Kharlampieva and co-workers that cubic capsules exhibited higher cell association than spherical capsules [34].
In this work, we reported the LbL assembly of cubic, tetradecahedral and spherical capsules using Cu2O particles as sacrificial templates. Cu2O particles can be removed under mild conditions (Na2S2O3 solution at neutral pH) without introducing toxic reagents. The capsules could maintain the template shapes after template removal and exhibit negligible cytotoxicity to HeLa cells. In addition, the capsule shape significantly influenced cell association, which cubic capsules significantly increased HeLa cell association compared to tetradecahedral and spherical capsules. The reported Cu2O particles with different shapes can serve as alternative templates for the engineering of polymer capsules with tunable morphologies for control over cell association, which provides a new prospect for the investigation of bio-nano interactions.
AcknowledgmentsThis work is financially supported by research general programs from the National Natural Science Foundation of China (Nos. 21603120, 21872085) and Fundamental Research Funding of Shandong University (No. 2017JC003).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.049.
| [1] |
G. Decher, Science 277 (1997) 1232-1237. DOI:10.1126/science.277.5330.1232 |
| [2] |
J.J. Richardson, M. Björnmalm, F. Caruso, Science 348 (2015) aaa2491. DOI:10.1126/science.aaa2491 |
| [3] |
X.L. Zhang, Y. Xu, X. Zhang, et al., Prog. Polym. Sci. 89 (2018) 76-107. |
| [4] |
J.J. Richardson, J. Cui, M. Bjornmalm, et al., Chem. Rev. 116 (2016) 14828-14867. DOI:10.1021/acs.chemrev.6b00627 |
| [5] |
B. Xue, V. Kozlovskaya, E. Kharlampieva, J. Mater. Chem. B 5 (2017) 9-35. DOI:10.1039/C6TB02746F |
| [6] |
M.J. Xuan, J. Zhao, J.X. Shao, et al., J. Colloid Interface Sci. 487 (2017) 107-117. DOI:10.1016/j.jcis.2016.10.018 |
| [7] |
V. Kozlovskaya, B. Xue, E. Kharlampieva, Macromolecules 49 (2016) 8373-8386. DOI:10.1021/acs.macromol.6b01740 |
| [8] |
O. Shimoni, Y. Yan, Y.J. Wang, F. Caruso, ACS Nano 7 (2012) 522-530. |
| [9] |
Y. Liu, J.F. Tan, A. Thomas, D.O. Yang, V.R. Muzykantov, Ther. Deliv. 3 (2012) 181-194. DOI:10.4155/tde.11.156 |
| [10] |
C. Kinnear, T.L. Moore, L.R. Rodriguez-Lorenzo, B.R. Rutishauser, A.P. Fink, Chem. Rev. 117 (2017) 11476-11521. DOI:10.1021/acs.chemrev.7b00194 |
| [11] |
J. Chen, V. Kozlovskaya, A. Goins, et al., Biomacromolecules 14 (2013) 3830-3841. DOI:10.1021/bm4008666 |
| [12] |
B. Xue, V. Kozlovskaya, F. Liu, et al., ACS Appl. Mater. Interfaces 7 (2015) 13633-13644. DOI:10.1021/acsami.5b03360 |
| [13] |
J.B. Song, X.Y. Yang, O. Jacobson, et al., Adv. Mater. 27 (2015) 4910-4917. DOI:10.1002/adma.201502486 |
| [14] |
X.H. Huang, M.M. Zhang, X.W. Dou, et al., Chin. Chem. Lett. 26 (2015) 1155-1157. DOI:10.1016/j.cclet.2015.04.005 |
| [15] |
Y.J. Wang, A.S. Angelatos, F. Caruso, Chem. Mater. 20 (2007) 848-858. |
| [16] |
L. Cui, R. Wang, X.Q. Ji, et al., Mater. Chem. Phys. 148 (2014) 87-95. DOI:10.1016/j.matchemphys.2014.07.016 |
| [17] |
J. Shi, J.W. Teng, Y.D. Wang, Y. Tang, Z.K. Xie, Chin. Chem. Lett. 26 (2015) 1409-1414. DOI:10.1016/j.cclet.2015.06.001 |
| [18] |
D. Luo, R.N. Poston, D.J. Gould, G.B. Sukhorukov, Mater. Sci. Eng. C 94 (2019) 647-655. DOI:10.1016/j.msec.2018.10.031 |
| [19] |
A. Yashchenok, B. Parakhonskiy, S. Donatan, et al., J. Mater. Chem. B 1 (2013) 1201-1372. |
| [20] |
N.G. Balabushevich, A.V.L. Guerenu, N.A. Feoktistova, A.G. Skirtach, D. Volodkin, Macromol. Biosci. 16 (2016) 95-105. DOI:10.1002/mabi.201500243 |
| [21] |
A.Q. Alorabi, M.D. Tarn, M. Thomas, V.N. Paunov, N. Pamme, Anal. Methods 10 (2018) 5335-5340. DOI:10.1039/C8AY02012D |
| [22] |
R. Kurapati, A.M. Raichur, J. Mater. Chem. B 1 (2013) 3175-3184. DOI:10.1039/c3tb20192a |
| [23] |
P. Wang, R.K. Kankala, J.Q. Fan, et al., J. Mater. Sci-Mater. Med. 29 (2018) 68. DOI:10.1007/s10856-018-6075-z |
| [24] |
M. Bagherzadeh, N. Mousavi, M. Amini, et al., Chin. Chem. Lett. 28 (2017) 1125-1130. DOI:10.1016/j.cclet.2017.01.022 |
| [25] |
X.W. Huang, Z.J. Liu, Y.F. Zheng, Chin. Chem. Lett. 22 (2011) 879-882. DOI:10.1016/j.cclet.2010.12.032 |
| [26] |
Y. Shang, D.F. Zhang, L. Guo, J. Mater. Chem. 22 (2012) 856-861. DOI:10.1039/C1JM14258E |
| [27] |
W.C. Huang, L.M. Lyu, Y.C. Yang, M.H. Huang, J. Am. Chem. Soc. 134 (2012) 1261-1267. DOI:10.1021/ja209662v |
| [28] |
D.F. Zhang, H. Zhang, L. Guo, et al., J. Mater. Chem. 19 (2009) 5220-5525. DOI:10.1039/b816349a |
| [29] |
J.W. Nai, Y. Tian, X. Guan, L. Guo, J. Am. Chem. Soc. 135 (2013) 16082-16091. DOI:10.1021/ja402751r |
| [30] |
J.D. Pillai, S.S. Dunn, M.E. Napier, J.M. DeSimone, IUBMB Life 63 (2011) 596-606. DOI:10.1002/iub.497 |
| [31] |
N. Doshi, B. Prabhakarpandian, A. Rea-Ramsey, et al., J. Control Release 146 (2010) 196-200. DOI:10.1016/j.jconrel.2010.04.007 |
| [32] |
O. Shchepelina, M.O. Lisunova, I. Drachuk, V.V. Tsukruk, Chem. Mater. 24 (2012) 1245-1254. DOI:10.1021/cm202820r |
| [33] |
S. Dasgupta, T. Auth, G. Gompper, Nano Lett. 14 (2014) 687-693. DOI:10.1021/nl403949h |
| [34] |
J.F. Alexander, V. Kozlovskaya, J. Chen, et al., Adv. Healthc. Mater. 4 (2015) 2657-2666. DOI:10.1002/adhm.201500537 |
 2020, Vol. 31
2020, Vol. 31 

