b School of Material Science and Engineering, Jilin Jianzhu University, Changchun 130118, China
The application of hydrophilic polymers as different carriers is blossoming with the diversification of polymerization techniques and development of potent synthetic approaches, leading to welldefined and various macromolecular architectures [1, 2]. In the past decades, the behavior of these synthetic polymers is particularly appealing since they can self-assemble in solution into nanometer-sized objects such as micelles, vesicles or wormlike micelles, depending on the polymer chemical composition and architecture [3-8]. Among these nano-objects, polymeric nanoparticles com-prising of hydrophilic polymers have drawn great attention owing to their attractive features such as ease of synthesis, tunable particle size, and controllable degradability [9-12]. Polymeric nanoparticles have exhibited great potential in numerous appli-cations including drug delivery, gene therapy, emulsification processes, particle stabilization and dispersion or catalyst organic supports [13-16]. Cross-linking a single polymer chain within itself can lead to small polymeric nanoparticles with well-defined properties [17]. Alternative strategy is utilizing polymers in conjunction with other functional motifs, which results in the creation of larger polymeric micro-or nanospheres driving by noncovalent interactions, e.g., electrostatic interaction, dipole-dipole interaction or hydrogen bonding [18-22]. Hydrogen bonding is a robust noncovalent interaction which has been successfully utilized to construct a series of superamolecular system in diverse forms [23-26]. It has turned up to be a novel and efficient strategy to construct assemblies these systems based on the hydrogen bonding between weak polyacids and neutral polymers bearing ether, ester or amide moieties [27].
Hydrophilic poly(2-oxazoline)s (POx) are a polymer class with diverse applications because of their great promise exhibited as biomaterials and thermo-responsive materials, as well as the easy access to defined amphiphilic structures for (hierarchical) self-assembly [28-38]. C=O moieties in the POx chain are potential hydrogen acceptors that can be utilized to build stable multilayers in combination with polyalcohols containing hydrogen donors [39, 40]. Very recently, our group reported the coassembly of POx and hydrogen donor, i.e., tannic acid (TA) into stable nanoparticles through hydrogen bonding in ethanol or water. The prepared nanoparticles were found to exhibit thermo-responsive properties in both water and ethanol [41].
Doxorubicin hydrochloride (Dox), one of the most effective agents for tumor therapy, has been widely used in clinical applications of anticancer therapy [42]. However, it can be quickly dissolved in tissue fluid owing to its high solubility, which could lead to initial burst release [43]. In addition, high concentrations of drugs can lead to toxic side effects, which are harmful to patients [44]. Therefore, it is of significance to seek appropriate carriers to improve the effectiveness of drug therapies and reduce the side effects. In view of the presence of hydroxyl, amino and carbonyl groups which are potential hydrogen donors and acceptors, we anticipated that Dox molecules may take part in the coassembly and form polymeric assemblies with POx and TA driving by the potential hydrogen bonding between different functionalities. Herein, we report the coassembly behavior of PMeOx, TA and Dox, leading to uniform and polymeric nanoparticles. We further systematically examine the toxicity of the hybrid nanoparticles as well as the releasing behavior of Dox in vitro. This work represents a simple and efficient strategy in providing stable platform for the development of efficient drug delivery carriers towards cancer treatment.
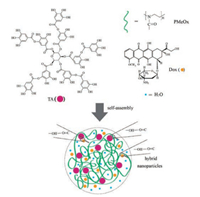
The coassembly of poly(2-oxazoline)-tannic acid polymeric nanoparticles has been demonstrated in our previous report, i.e., direct mixing of POx and TA aqueous solutions gave assembled nanoparticles with defined diameter and stability by means of the hydrogen bonding between POx and TA [41]. Prior to the coassembly of these two molecules with Dox, we examine the assembly behavior of Dox/POx and Dox/TA. The addition of poly(2-methyl-2-oxazoline) (PMeOx) or TA aqueous solution to Dox aqueous solution all gave transparent solutions. Thus, the coassembly between Dox and POx/TA are ruled out. To avoid the dominant assembly between POx and TA, and further ensure a well-distribution of Dox in the assemblies, the PMeOx aqueous solution was added to the premixed aqueous solution containing TA and Dox. The solution turned to be turbid after the addition of PMeOx, indicating the formation of PMeOx-TA-Dox assemblies. Successively, free polymer and small molecules were removed by dialysis. The molecular formula and the assembly path of PMeOx, TA and Dox are schematically outlined in Scheme 1.
|
Download:
|
| Scheme 1. Chemical structures of tannic acid (TA), poly(2-methyl-2-oxazoline) (PMeOx) and doxorubicin (Dox), and the schematic illustration of their assembly into nanoparticles in water. | |
Successively, the morphology and size of the PMeOx-TA-Dox assemblies at a weight ratio of PMeOx-TA and Dox of 3:4 were examined by scanning electron microscopy (SEM). As shown in Fig. 1A, the three-component coassembly afforded uniform nanoparticles with an average diameter of 132 nm. Moreover, an average diameter of 143 nm as determined by dynamic light scattering (DLS) measurement was obtained (Fig. 1B), which is comparable with the sized that determined by SEM. Stability of nanoparticles is a prerequisite for further drug loading and delivery. Therefore, the assembled nanoparticles in aqueous solution were monitored by DLS at different time. As shown in Fig. 1B, the diameter of PMeOx-Dox-TA only varied slightly after reaching the equilibrium, and kept almost constant for within 20 days. Furthermore, the negligible change of particle size after storage in phosphate-buffered saline (PBS, pH 7.4) containing 10% fetal bovine serum (FBS) for 6 days suggested that the assembled nanoparticles possessed a good stability in physiological environ-ment.

|
Download:
|
| Fig. 1. SEM image (A) and size distribution diagram determined by DLS (B) of PMeOx-Dox-TA at a weight ratio of PMeOx-TA : Dox of 4 : 3 (inset in B: diameter of the assembled nanoparticle as a function of the storage period). | |
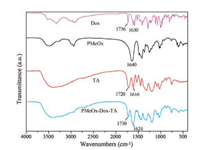
The formation of PMeOx-Dox-TA nanoparticles is presumed to the noncovalent interaction, i.e., hydrogen bonding occurred between POx, TA and Dox, according to previous report [41]. To verify the presence of hydrogen bonding between the functionali-ties within the PMeOx-TA-Dox assemblies, infrared spectroscopy (IR) characterization was conducted after the removal of water by freeze-drying the nanoparticle dispersion solution. As shown in Fig. 2, the intensive band at 1642 cm-1 assigned to the carbonyl stretching mode is typical for PMeOx. After the formation of nanoparticles, this peak shifted to lower wavenumbers at 1632 cm-1 due to the presence of hydrogen bonding between -C=O from POx and hydrogen bond donor from TA or Dox [45]. Moreover, the intensive band at 1723 cm-1 originated from C=O stretching mode of TA all shifted to 1732 cm-1 after the formation of assemblies, indicating the formation of inter-or intramolecular hydrogen bond between the C=O group and the hydrogen bond donor [45]. In view of the presence of -OH and -COOH vibration modes in the IR spectrum, not all functionalities were involved in the formation of hydrogen bonding. Moreover, electrostatic interaction among the nanoparticles cannot be ruled out due to the presence of COO- and H+ ions, etc.
|
Download:
|
| Fig. 2. IR spectra of Dox, PMeOx, TA and PMeOx-Dox-TA. | |
In order to achieve the efficient encapsulation of Dox into the assembled polymeric nanoparticles, the assembly conditions were screened and optimized in terms of concentration and ratio of Dox to PMeOx and TA. For PMeOx-TA assembled nanoparticles, the optimum assembled nanoparticles could be obtained when the amount of OH from TA and the amount of -C=O from PMeOx were kept stoichiometric equivalent according to our previous report [41]. Therefore, the feeding amount of OH from TA and the amount of -C=O from PMeOx were always kept identical, and the feeding weight content of Dox was kept at 10 wt% for PMeOx-TA-Dox assembly. Initially, we investigated the concentration effect analogously to the previous report. Increasing the concentration from 0.05 μg/mL to 2.0 μg/mL resulted in a diameter increase from 60 nm to 215 nm for PMeOx-TA-Dox nanoparticles as determined by DLS measurement.
Although increasing the weight ratio of Dox to PMeOx and TA can slightly enhance the drug loading efficiency (DLE), which might be attributed to less amount hydrogen donor/acceptor possessed by Dox in comparison to that for PMeOx and TA. When the weight ratio of Dox is above 14%, no assembled nanoparticles could be formed. Although the DLE could not be above 13.3% via the optimization, our investigation provides a very simple method by direct mixing the aqueous solution without the utilization of any organic solvents.
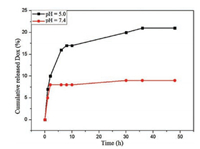
A sustained-release character is essential for an efficient drug delivery [46]. The drug release behavior of PMeOx-TA-Dox was evaluated in PBS solutions at different pH values (pH 5.0 and 7.4). Fig. 3 shows the release profiles of the Dox at pH 5.0 and 7.4 at 37 ℃. The release rate of Dox at pH 5.0 is significantly higher than that at pH 7.4. Such a release behavior of Dox is important, because the pH values in tumor extracellular, endosomes and lysosomes is more acidic (pH 5.0–6.5) than that for blood (pH 7.4) [47-50], which is beneficial for cancer therapy [51].
|
Download:
|
| Fig. 3. Dox release profiles of PMeOx-Dox-TA in PBS (pH 5.0 and 7.4, 20 mmol/L). | |
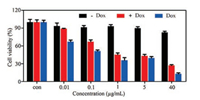
The biocompatibility of nanomaterials is of great significance for their applications in biomedical fields, thus the cytotoxicity of assembled nanoparticles of PMeOx and TA toward human epithelial carcinoma cells (HeLa cells lines) was firstly evaluated by a standard 3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyl tetrazo-lium bromide (MTT) assay [52, 53]. As shown in Fig. 4, no obvious cytotoxicity was observed even at a concentration of 10 μg/mL for PMeOx-TA after an incubation time of 48 h, indicating an excellent biocompatibility of PMeOx-TA nanoparticles. Then, the cytotox-icities of PMeOx-TA-Dox and free Dox against HeLa cells determined by MTT assays after 48 h culture were conducted, and the results are shown in Fig. 4. It can be observed that these cytotoxicities are all concentration dependent. The inhibition efficacy of PMeOx-TA-Dox is slightly lower than that of free Dox at lower concentrations (0.0001–0.4 μg/mL). However, it exhibited similar activities to free Dox at a concentration of 5.0 μg/mL. This result demonstrates that PMeOx-TA-Dox still possesses the ability to kill cancer cells.
|
Download:
|
| Fig. 4. In vitro biocompatibility of PMeOx-TA against HeLa cells (black), viabilities of HeLa cells incubated with free DOX (purple) and PMeOx-TA-Dox (red) for 48 h at 37 ℃. All the results were repeated three times and presented as mean ±SD. | |
To gain insight into the intracellular behavior of PMeOx-TA-Dox, we investigated their cellular uptake character by the inherent fluorescence of Dox using confocal laser scanning microscopy (CLSM). Human cervical carcinoma (HeLa) cells were chose for the evaluation. The cellular nuclei were selectively stained with 4', 6-diamidino-2-phenylindole (DAPI). CLSM images of HeLa cells after incubation with PMeOx-TA-Dox for 0.5 h, 2 h and 6 h are shown in Fig. 5. The cellular uptake of PMeOx-TA-Dox was then evaluated by CLSM. As can be seen from Fig. 5, nearly no fluorescence of Dox could be detected at 30 min, and only weak red fluorescence could be observed in the cytoplasm at 2 h. While after 6 h incubation, bright red fluorescence could be observed clearly both in the cellular nuclei and cytoplasm of HeLa cells.

|
Download:
|
| Fig. 5. CLSM images of HeLa cells incubated with PMeOx-TA-Dox for different time periods at 37 ℃ at a Dox concentration of 0.2 μg/mL. Cells are viewed in the blue channel for DAPI and in the red channel for Dox. Scale bars represent 25 μm in all images. | |
In conclusion, we have developed a facile strategy in the straightforward preparation of supramolecular hybrids via hydrogen bonding and the electrostatic interactions among the PMeOx, TA and Dox. The prepared spherical PMeOx-TA-Dox nanoparticles are water dispersible and exhibit high stability and good biocompatibility. The pH-dependent loading and release behaviors and the cellular uptake property enable the prepared nanoparticles as promising candidate as drug carriers for therapy. Our proof-ofconcept strategy and the incorporation of drug molecules into the assemblies represent an attractive approach in the preparation of nanomedicine. Further optimization of loading efficiency and the application in vivo is under investigation in our lab.
AcknowledgmentsThe financial support of this work from the National Natural Science Foundation of China (No. 51673194), Department of Science and Technology of Jilin Province (Nos. 20180101196JC and 20180101170JC) is greatly acknowledged.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.041.
| [1] |
C. Giacomelli, V. Schmidt, K. Aissou, R. Borsali, Langmuir 26 (2010) 15734-15744. DOI:10.1021/la100641j |
| [2] |
G. Riess, Prog. Polym. Sci. 28 (2003) 1107-1170. DOI:10.1016/S0079-6700(03)00015-7 |
| [3] |
O. Ikkala, G. ten Brinke, Science 295 (2002) 2407-2409. DOI:10.1126/science.1067794 |
| [4] |
J. Du, H. Willcock, J.P. Patterson, I. Portman, R.K. O'Reilly, Small 7 (2011) 2070-2080. DOI:10.1002/smll.201100382 |
| [5] |
C. Bouilhac, E. Cloutet, D. Taton, et al., Macromolecules 41 (2008) 7321-7329. DOI:10.1021/ma8016772 |
| [6] |
C. Bouilhac, E. Cloutet, D. Taton, et al., J. Polym. Sci. Part A Polym. Chem. 47 (2009) 197-209. DOI:10.1002/pola.23142 |
| [7] |
K.M. Zepon, I. Otsuka, C. Bouilhac, et al., Biomacromolecules 16 (2015) 2012-2024. DOI:10.1021/acs.biomac.5b00443 |
| [8] |
G. Lopez, M. Guerre, J. Schmidt, et al., Polym. Chem. 7 (2016) 402-409. DOI:10.1039/C5PY01621E |
| [9] |
J.P. Rao, K.E. Geckeler, Prog. Polym. Sci. 36 (2011) 887-913. DOI:10.1016/j.progpolymsci.2011.01.001 |
| [10] |
C.J. Hawker, K.L. Wooley, Science 309 (2005) 1200-1205. DOI:10.1126/science.1109778 |
| [11] |
C. Vauthier, K. Bouchemal, Pharm. Res. 26 (2009) 1025-1058. DOI:10.1007/s11095-008-9800-3 |
| [12] |
C.A. Figg, A. Simula, K.A. Gebre, et al., Chem. Sci. 6 (2015) 1230-1236. DOI:10.1039/C4SC03334E |
| [13] |
A.K. Boal, F. Ilhan, J.E. DeRouchey, et al., Nature 404 (2000) 746-748. DOI:10.1038/35008037 |
| [14] |
S.S. Guterres, M.P. Alves, A.R. Pohlmann, Drug Target Insights 2 (2007) 147-157. |
| [15] |
R.A. Petros, J.M. DeSimone, Nat. Rev. Drug Discov. 9 (2010) 615-627. DOI:10.1038/nrd2591 |
| [16] |
H. Yang, B. Yuan, X. Zhang, O.A. Scherman, Acc. Chem. Res. 47 (2014) 2106-2115. DOI:10.1021/ar500105t |
| [17] |
B.T. Tuten, D. Chao, C.K. Lyon, E.B. Berda, Polym. Chem. 3 (2012) 3068-3071. DOI:10.1039/c2py20308a |
| [18] |
Y. Wang, S.A. Sukhishvili, Macromol. Rapid Commun. 38 (2017) 1700242. DOI:10.1002/marc.201700242 |
| [19] |
O. Altintas, C. Barner-Kowollik, Macromol. Rapid Commun. 33 (2012) 958-971. DOI:10.1002/marc.201200049 |
| [20] |
M. Seo, B.J. Beck, J.M.J. Paulusse, C.J. Hawker, S.Y. Kim, Macromolecules 41 (2008) 6413-6418. DOI:10.1021/ma8009678 |
| [21] |
M. Elsabahy, K.L. Wooley, Chem. Soc. Rev. 41 (2012) 2545-2561. DOI:10.1039/c2cs15327k |
| [22] |
Z. Liu, Y. Jiao, Y. Wang, C. Zhou, Z. Zhang, Adv. Drug Deliv. Rev. 60 (2008) 1650-1662. DOI:10.1016/j.addr.2008.09.001 |
| [23] |
T. Steiner, Angew. Chem. Int. Ed. 41 (2002) 48-76. DOI:10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U |
| [24] |
T. Kato, N. Mizoshita, K. Kishimoto, Angew. Chem. Int. Ed. 45 (2005) 38-68. |
| [25] |
O. Ikkala, G. ten Brinke, Science 295 (2002) 2407-2409. DOI:10.1126/science.1067794 |
| [26] |
X.Z. Yan, F. Wang, B. Zheng, F.H. Huang, Chem. Soc. Rev. 41 (2012) 6042-6065. DOI:10.1039/c2cs35091b |
| [27] |
I. Erel-Unal, S.A. Sukhishvili, Macromolecules 41 (2008) 3962-3970. DOI:10.1021/ma800186q |
| [28] |
N. Adams, U.S. Schubert, Adv. Drug Deliv. Rev. 59 (2007) 1504-1520. DOI:10.1016/j.addr.2007.08.018 |
| [29] |
L. Hou, J. Fang, W. Wang, et al., J. Mater. Chem. B 5 (2017) 3348-3354. DOI:10.1039/C7TB00812K |
| [30] |
F.C. Gaertner, R. Luxenhofer, B. Blechert, R. Jordan, M. Essler, J. Control. Release 119 (2007) 291-300. DOI:10.1016/j.jconrel.2007.02.015 |
| [31] |
N. Zhang, S. Huber, A. Schulz, et al., Macromolecules 42 (2009) 2215-2221. DOI:10.1021/ma802627y |
| [32] |
R. Konradi, B. Pidhatika, A. Mühlebach, M. Textor, Langmuir 24 (2008) 613-616. DOI:10.1021/la702917z |
| [33] |
N. Zhang, T. Pompe, I. Amin, et al., Macromol. Biosci. 12 (2012) 926-936. DOI:10.1002/mabi.201200026 |
| [34] |
C. Taubmann, R. Luxenhofer, S. Cesana, R. Jordan, Macromol. Biosci. 5 (2005) 603-612. DOI:10.1002/mabi.200500059 |
| [35] |
S. Kobayashi, H. Uyama, J. Polym. Sci. Part A Polym. Chem. 40 (2002) 192-209. |
| [36] |
R. Hoogenboom, F. Wiesbrock, M.A.M. Leenen, et al., Macromolecules 40 (2007) 2837-2843. DOI:10.1021/ma062725e |
| [37] |
N. Zhang, M. Steenackers, R. Luxenhofer, R. Jordan, Macromolecules 42 (2009) 5345-5351. DOI:10.1021/ma900329y |
| [38] |
R. Luxenhofer, M. Bezen, R. Jordan, Macromol. Rapid Commun. 29 (2008) 1509-1513. DOI:10.1002/marc.200800293 |
| [39] |
A.B. da Fonseca Antunes, M. Dierendonck, G. Vancoillie, et al., Chem. Commun. 49 (2013) 9663-9665. DOI:10.1039/c3cc45068f |
| [40] |
I. Erel, H. Schlaad, A.L. Demirel, J. Colloid Interface Sci. 361 (2011) 477-482. DOI:10.1016/j.jcis.2011.05.033 |
| [41] |
C. Wang, Y. Li, Y. Ma, et al., J. Polym. Sci. A Polym. Chem. 56 (2018) 1520-1527. DOI:10.1002/pola.29033 |
| [42] |
S. Sanyakamdhorn, D. Agudelo, H.A. Tajmir-Riahi, Biomacromolecules 14 (2013) 557-563. DOI:10.1021/bm3018577 |
| [43] |
Y. Fu, W.J. Kao, Expert Opin. Drug Deliv. 7 (2010) 429-444. DOI:10.1517/17425241003602259 |
| [44] |
M. Schulz, S. Iwersen-Bergmann, H. Andresen, A. Schmoldt, Crit. Care 16 (2012) R136. DOI:10.1186/cc11441 |
| [45] |
H. Fan, L. Wang, X. Feng, et al., Macromolecules 50 (2017) 666-676. DOI:10.1021/acs.macromol.6b02106 |
| [46] |
J. Park, T.F. Brust, H.J. Lee, et al., ACS Nano 8 (2014) 3347-3356. DOI:10.1021/nn405809c |
| [47] |
X. Hu, X. Guan, J. Li, et al., Chem. Commun. 50 (2014) 9188-9191. DOI:10.1039/C4CC04056B |
| [48] |
M. Meyer, A. Philipp, R. Oskuee, C. Schmidt, E. Wagner, J. Am. Chem. Soc. 130 (2008) 3272-3273. DOI:10.1021/ja710344v |
| [49] |
J.Z. Du, T.M. Sun, W.J. Song, J. Wu, J. Wang, Angew. Chem. Int. Ed. 49 (2010) 3621-3626. DOI:10.1002/anie.200907210 |
| [50] |
Y. Lee, T. Ishii, H. Cabral, et al., Angew. Chem. Int. Ed. 48 (2009) 5309-5312. DOI:10.1002/anie.200900064 |
| [51] |
J. Bai, Y. Liu, X. Jiang, Biomaterials 35 (2014) 5805-5813. DOI:10.1016/j.biomaterials.2014.04.008 |
| [52] |
T. Sun, X. Guan, M. Zheng, X. Jing, Z. Xie, ACS Med. Chem. Lett. 6 (2015) 430-433. DOI:10.1021/acsmedchemlett.5b00041 |
| [53] |
L. Yang, Z. Wang, J. Wang, et al., Nanoscale 8 (2016) 6801-6809. DOI:10.1039/C6NR00247A |
 2020, Vol. 31
2020, Vol. 31 

