b Department of Chemistry, School of Science, Tianjin University, Tianjin 300000, China
Cancer is the leading cause of death and major public health problems worldwide [1]. In China, the morbidity and mortality of cancer continue to rise. Among them, lung cancer is the most common incident malignant tumor disease, accounting 20.55% of the total number of death [2, 3]. Thus, it is important to explore a high efficiency and low toxicity anti-lung cancer drugs. Due to the huge time and money cost of developing anti-tumor drugs, it is necessary to conduct new excavations on the pharmacological effects of existing drugs, which can save medical resources, improve the survival and life quality of patients. Traditional Chinese medicine (TCM) has been used to provide medical therapy in China from ancient time. With the deepening of research on cancer, many TCM and ethnic medicines have been found to induce tumor cell apoptosis and inhibit proliferation in tumor cells [4-7].
"Dai-Bai-Jie" is a traditional herbal medicine of the Dai nationality, which is the dry root of Marsdenia tenacissima (Roxb.) Moon (Asclepiadaceae) [8], and extensively distributed in the south of China. The main chemical component of "Dai-Bai-Jie" is polyoxypregnane glycosides, with a variety of biological activities and pharmacological effects [9-11]. It is commonly used in the detoxification, decreasing swelling, alleviating pain, and has significant curative effect on the treatment of various malignant tumors [12-15], by Dai people who living in Laos, Burma, and the Yunnan province of China. Dai-Bai-Jie is used by the main medicinal material for preparations such as Ya Jie Tablet and Baijie Capsule. However, most of the research on Dai-Bai-Jie focuses on the composition analysis and genus identification, but anti-tumor research is lacking.
In this study, we investigated the anti-tumor ability of the Dai-Bai-Jie water extracts. The effect of different concentration drug was explored on the proliferation, apoptosis and migration of A549 cells (Fig. 1). We found that as the concentration of drug increased, the activity of A549 cells decreased and apoptosis increased, and the ability of migration gradually decreased.
|
Download:
|
| Fig. 1. Schematic diagram of the traditional Chinese medicine Dai-Bai-Jie's effect on the proliferation and migration of A549 cells. | |
In all experiment: The roots of Marsdenia tenacissima (Roxb.) Moon (“Dai-Bai-Jie”) materials were purchased from Xishuang-banna, Yunnan province of China. Human lung tumor A549 cells were purchased from Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. Purified water was produced by Wahaha Group (Hangzhou, China). Cell Counting Kit-8(CCK8)-100 T was purchased from Dojindo Laboratories (Beijing, China). Fetal bovine serum was purchased from Beyotime Biotech (Shanghai, China). RPMI1640 medium was purchased from Grand Island (USA). Dihydroe-thidium was purchased from Be-yotime (Shanghai, China).
The A549 human lung adenocarcinoma cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 μg/mL penicillin and 100 μg/mL streptomycin. The A549 cells were maintained at 37 ℃, 5% CO2 incubator, and all cell growth state is consistent. The cells were passaged once every 3 days, and the medium was changed every other day, and the cells were used for experiments in the logarithmic growth phase.
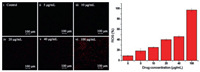
To evaluate the effect of traditional Chinese medicine on cell growth rate, A549 cells in the logarithmic growth phase were collected and diluted with a medium, followed by seeding to a 96-well plate, and the number of cells per well was about 3, 000. After 24 h, the medium containing different concentrations (0, 5, 10, 20, 40, 100 μg/mL) of Dai-Bai-Jie was added as the experimen-tal group, blank holes (medium without cells) and control holes (medium without drugs, with cells) were set, and three compound holes were set for each group. After 24 and 48 h, 10 μL CCK8 was added into per well and incubated at 37 C for 2 h, and then measure the absorbance (A) of each well at 450 nm using a microplate reader. Cell viability % = (add drug cell OD blank OD)/(control cell OD blank OD) 100%. Dai-Bai-Jie inhibited the proliferation of A549 cells (Fig. 2a). The inhibition rate of A549 cells increased with the concentration of Dai-Bai-Jie solution, and the longer the time, the stronger the inhibition, showing a time dependent. The IC50 (half maximal inhibitory concentration) value at 24 h and 48 h was 28.49 and 13.27 μg/mL, respectively, indicating a good inhibitory effect on cell proliferation (Fig. 2b).

|
Download:
|
| Fig. 2. Cytotoxicity of Dai-Bai-Jie on lung cancer cell A549 in vitro. (a) Cell Counting Kit-8 assay was performed to detect viability of Dai-Bai-Jie after 12, 24 and 48 h treatment in A549 cells; (b) IC50 values at different time. n = 3, ***P < 0.001. | |
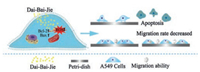
The cells were seeded in petri-dishes and incubated with different concentrations (0, 5, 10, 20, 40, 100 μg/mL) of Dai-Bai-Jie for 24 h. After washed with PBS, a live/dead assay kit composed of calcein AM was used to investigate the cell viability. Place the cells in the incubator for 20 min. The effect of Dai-Bai-Jie on apoptosis of A549 cells was observed by fluorescence microscope. It was found that the viability of living cells decreased gradually from the control group to the high-concentration group. It indicated that Dai-Bai-Jie could induce the apoptosis of A549 cells and reduce the survival rate of A549 cells (Fig. 3).
|
Download:
|
| Fig. 3. A live/dead assay kit composed of calcein AM and propidium iodide (PI) was used to investigate the cell viability after 24 h. | |
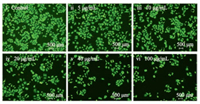
To investigate whether the apoptosis level and reactive oxygen species (ROS) level of cells were different with the above experimental results. We used ROS fluorescent probes to monitor cell changes (Fig. 4). The red color represented the intracellular ROS production level and the apoptosis (Fig. 4, left). The more red areas showed high ROS production. 2-(2, 7-Dichloro-3, 6-diacetyloxy-9H-xanthen-9-yl)-benzoic acid (DCFH-DA) solution (10 μmol/L) diluted with serum-free liquid was added into the logarithmic growth phase A549 cells, and incubated at 37 ℃ for 30 min, followed by washing two times with serum-free cell culture medium. Then a confocal microscope (Zeiss LSM780) was used to detect the ROS production. It was found that the fluorescence intensity of ROS in A549 cells was increased with the concentration of the drug (Fig. 4, right). When the drug concentration was 5 μg/mL, the intracellular ROS was not significantly changed, at 100 μg/mL the number of red dots increased and the fluorescence in the cells was obviously observed. The results above confirmed that Dai-Bai-Jie could promote the cellular damage, and increased drug toxicity with the concentra-tion increasing.
|
Download:
|
| Fig. 4. A549 intracellular ROS generation with increase of Dai-Bai-Jie concentration. Left: Cell imaging, Right: ROS responses. | |
The occurrence and development of tumors are closely related to the infinite proliferation of tumor cells and apoptosis [16-18]. Tumor recurrence and metastasis are related to the migration ability of tumor cells themselves. Scratch test can simulate the process of tumor cell metastasis. We collected A549 cells in logarithmic growth phase, and incubated the cells in a six-well plate. When the cells were about 90% spread over the petri dish, a 200 μL sterile tip was used to draw a straight line along the center of the well plate. After washing with PBS, different concentrations (0, 5, 10, 20, 40, 100 μg/mL) of Dai-Bai-Jie were added. The serum-free medium was cultured in groups. Photographs were taken with a microscope at 0 and 48 h, and the scratch width was calculated using Image J software (n = 3). Move the scratch width of the shift rate = (1 L48/L0)×100%, L48 and L0 are the scratch width at 48 h and 0 h, respectively. The cell migration was observed by microscopy at 0 h and 48 h after treatment with different concentrations of Dai-Bai-Jie (Fig. 5, left). The migration ability of A549 cells decreased after the action of Dai-Bai-Jie, with the increase of concentration, the mobility of cells gradually decreases (Fig. 5, right). This indicates that Dai-Bai-Jie solution inhibits the migration of A549 cells in a concentration-dependent manner.

|
Download:
|
| Fig. 5. The cell migration ability on lung cancer cell A549 in vitro from 0 h to 48 h. Left: Cell imaging; Right: Cell migration. | |
In summary, we found that Dai-Bai-Jie could inhibit the proliferation, migration of A549 human lung cancer cells, and promote the cell apoptosis. This work will supply inspirations to researchers working on Dai-Bai-Jie anti-tumor effect. In the future, we will study the in vivo anti-cancer effect of Dai-Bai-Jie and study its active components.
AcknowledgmentsThis work was supported by the National Key R & D Program of China (No. 2017YFC0906800) and National Natural Science Foundation of China (Nos. 21435002 and 21621003).
| [1] |
R.L. Siegel, K.D. Miller, A. Jemal, CA Cancer J. Clin. 1 (2016) 7-30. DOI:10.3322/caac.21349 |
| [2] |
W. Chen, R. Zheng, P.D. Baade, et al., CA Cancer J. Clin. 2 (2015) 115-132. DOI:10.3322/caac.21338 |
| [3] |
W. Chen, R. Zheng, H. Zeng, S. Zhang, Thorac. Cancer 6 (2015) 209-215. DOI:10.1111/1759-7714.12169 |
| [4] |
W. Xu, G. Yang, Y. Xu, et al., Evid-Based. Compl. Alt. 278 (2014) 917-926. DOI:10.1155/2014/278917 |
| [5] |
V.B. Konkimalla, E. Thomas, J. Ethnopharmacol. 116 (2008) 207-210. DOI:10.1016/j.jep.2007.12.009 |
| [6] |
G.M. Cragg, D.J. Newman, J. Ethnopharmacol. 100 (2005) 72-79. DOI:10.1016/j.jep.2005.05.011 |
| [7] |
J. Khazir, B.A. Mir, L. Pilcher, D.L. Riley, Phytochem. Lett. 7 (2014) 173-181. DOI:10.1016/j.phytol.2013.11.010 |
| [8] |
H.T. Li, L.P. Kang, B.L. Guo, et al., Chin. J. Chin. Mater. Med. 39 (2014) 1525-1529. |
| [9] |
X. Pang, L. Kang, H. Yu, et al., Steroids 93 (2015) 68-76. DOI:10.1016/j.steroids.2014.11.004 |
| [10] |
J.L. Li, J. Zhou, Z.H. Chen, et al., J. Nat. Prod. 78 (2015) 1548-1555. DOI:10.1021/np501058b |
| [11] |
S.X. Qiu, S.Q. Luo, L.Z. Lin, G.A. Cordell, Phytochemistry 41 (1996) 1385-1388. DOI:10.1016/0031-9422(95)00770-9 |
| [12] |
W. Fan, L. Sun, J.Q. Zhou, et al., Chin. J. Nat. Med. 13 (2015) 428-437. |
| [13] |
S.Y. Han, W. Zhao, H. Sun, et al., Phytomedicine 22 (2015) 560-567. DOI:10.1016/j.phymed.2015.03.001 |
| [14] |
Y. Wang, B. Chen, Z. Wang, et al., Oncotarget 13 (2016) 82851-82863. DOI:10.18632/oncotarget.7965 |
| [15] |
M.Q. Li, J.H. Shen, B. Xu, J. Chen, J. Interven. Radiol. 10 (2001) 228-231. |
| [16] |
G. Gobe, M. Rubin, G. Williams, I. Sawczuk, R. Buttyan, Cancer Inves. 20 (2002) 324-332. DOI:10.1081/CNV-120001177 |
| [17] |
D. Capuzzi, E. Santoro, W.W. Hauck, et al., Cancer 88 (2000) 24-34. DOI:10.1002/(SICI)1097-0142(20000101)88:1<24::AID-CNCR5>3.0.CO;2-W |
| [18] |
S.Y. Han, M.B. Zhao, G.B. Zhuang, P.P. Li, Lung Cancer 75 (2012) 30-37. DOI:10.1016/j.lungcan.2011.06.001 |
 2020, Vol. 31
2020, Vol. 31 

