b Department of Medical Oncology, The First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, China
Thyroid-stimulating hormone (TSH), a glycoprotein secreted by thyrotroph cells of the anterior pituitary gland, affects the endocrine function of the thyroid gland [1]. TSH levels typically fall between 0.4 μIU/L and 4.0 μIU/L (μIU/L: milliunits per liter) according to the American Thyroid Association. Higher TSH levels may lead to some diseases such as benign tumor of the pituitary, thyroid hormone carcinoma, congenital hypothyroidism and Hashimoto's thyroiditis, while lower TSH levels may cause hypopituitarism and Graves' disease [2]. Thus, a simple and sensitive method for detection of TSH in biological fluids is very crucial in clinical diagnostics and treatment. Immunological methods have become the predominant analytical tools for quantitative monitoring of disease-related proteins [3, 4]. A potentiometric immunosensor, based on the change of the membrane potential, is the most straightforward strategy to monitor the target analyte without the requirement of the external excitement (e.g., current and voltage) during the measurement [5]. Typically, the detection signal often derives from the electrical double layer after biological binding as a result of the variation in the electrical potential [6]. Such an electrical double layer affects significantly behaviors of colloids and other surfaces in contact with solution or solid-state fast ion conductors [7]. Because of the portability and easy operation, potentiometric immunosensor can be used as a transducer for TSH detection.
To achieve a high assay sensitivity, the signal amplification is important for the development of potentiometric immunosensor, especially for detection of low-level proteins. Methods based on potentiometer have been reported for the immunoassays by using an indicator system with different signal amplification strategies [8-10]. Routine approaches often consist of enzyme labels or nano labels. Undoubtedly, enzyme labels are used more widely than any other labels because one enzyme molecule can cause the conversion of numerous enzymatic substrates per min [11]. Generally, membrane potential of potentiometric immunosensor mainly consists of surface potential (i.e., Donnan potential) and diffusion potential [12]. Under conditions of constant electrolyte concentration on both sides of the membrane, the potential relies on the charge density on the membrane and the ionic transport capacity. Favorably, the Tang's group reported that horseradish peroxidase (HRP) could oxidize 4-chloro-1-naphthol (4-CN) into an insoluble benzo-4-chlorohexadienone via the biocatalytic precipitate technique [13-16]. More inspiringly, the as-generated precipitate is a species of uncharged organic substrate with weak conductivity, thus heavily decreasing the membrane potential with the coating on the sensor surface. However, the catalytic efficiency of single HRP molecule is always limited toward the substrate. In contrast, the emergence of the bionanotechnology opens a new horizon for the signal amplification [17]. Liposome with highly versatile lipid bilayer structure attracts greatest attention due to its high encapsulation capacity toward different signaling tags, e.g., enzyme, dye and quantum dots. Significantly, the entrapped liposomes are easily lysed through the hydrolytic agents or surfactant, thereby causing the releasing of numerous indicators from the encapsulated nanovehicles [18, 19]. To this end, our motivation in this work is to use enzyme-encapsulated liposome with enzymatic catalytic precipitation technique for the signal amplification.
Herein, we report the proof-of-concept of simple and portable potentiometric immunosensing protocol for sensitive detection of TSH by using enzyme-encapsulated liposome with enzymatic biocatalytic precipitation (Scheme 1). This system consists of immunosensor preparation, immunoreaction, liposome lysis and potentiometric measurement. Detailed information can be found in Supporting information. To construct such an assay system, monoclonal mouse anti-human TSH antibody (mAb1) is initially modified on an activated glassy carbon electrode (mAb1-GCE) using covalent conjugation with N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethyleaminopropy)carbodiimide hydrochloride (EDC). Biotinylated liposome encapsulated with HRP molecules by using the reverse-phase evaporation method is utilized for the conjugation of biotinylated polyclonal rabbit anti-human TSH secondary antibody (pAb2) via a typical biotin-avidin linkage. In the presence of target TSH, a sandwich-type immunoreaction is carried out between the immobilized mAb1 and the biotinylated pAb2. Accompanying formation of immunocomplex, the HRP-encapsulated liposome (HRPEL) is conjugated onto the electrode.Upon Triton X-100 introduction, the biofunctionalized liposome is lysed to release the entrapped HRP molecules, which can catalyze the 4-CN into an insoluble benzo-4-chlorohexadienone in the presence of H2O2 and coat on the surface of the electrode. Since benzo-4-chlorohexadienone is an insoluble and uncharged pre-cipitate, the coated chemicals cause the change of the modified electrode in the membrane potential relative to background signal. The shift in the potential (mV) can be determined on a portable potentiometer, which is proportional to the concentration of target TSH in the sample. Introduction of liposome in this system is expected to enhance the sensitivity of potentiometric immuno-sensor.
|
Download:
|
| Scheme 1. Schematic illustration of potentiometric immunosensor for the detection of thyroid-stimulating hormone (TSH) on monoclonal mouse anti-human TSH antibody (mAb1)-modified glassy carbon electrode (GCE) by using horseradish peroxidase (HRP)-encapsulated liposome functionalized with biotin, based on HRP-catalyzed precipitation of 4-chloro-1-naphthol (4-CN). | |
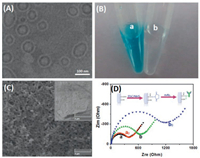
As described above, HRP molecules were encapsulated into the liposome by the reverse-phase evaporation method. In this case, the successful preparation of the liposome nanocontainers is very vital critical. Firstly, we used transmission electron microscopy (TEM; H-7650, Hitachi Instruments, Japan) to characterize the nanocarriers. As shown in Fig. 1A, the nanoliposomes displayed the spherical-like structures with an average size of ~120 nm in diameter. Also, we can observe that the thin shell became well-demarcated and had no rupture in the capsule, suggesting the formation of vesicle structure. Such a vesicle structure could be employed for the loading of numerous HRP molecules. To verify this issue, we might roughly estimate from a statistical point of view that the effective volume of one liposome was ~7.4×105 nm3 (note: The calculation is implemented on the basis of the assumption of spherical volume, V = 4/3 πr3, where r stands for the effective radius of liposome; and the thickness of a lipid bilayer is ~4.0 nm)[20-22]. One liposome could accommodate 6.5×103 HRP molecules at least (note: The calculation referred to the effective volume of liposome divided by the volume of one HRP molecule with a molecular radius of 30 Å, ~3.0 nm).Logically, one puzzling question arises as to whether HRP molecules were readily encapsulated into the liposome. To demonstrate this point, biotinylated liposomes with and without HRP molecules were employed to catalyze the colorimetric reaction relative to typical TMB-H2O2 system (TMB: 3, 3', 5, 5'-tetramethylbenzidine) (Fig. 1B). Obviously, the blue color was appeared only if HRP was present in the liposome (sample a), and biotinylated liposomes alone without HRP molecule could not cause the color change of TMB-H2O2 system (sample b). These results indicated that HRP molecules were encapsulated into the liposome with successful preparation.
|
Download:
|
| Fig. 1. (A) TEM image of HRP-encapsulated liposome, scale bar: 100 nm; (B) photographs of (a) HRP-encapsulated liposome and (b) liposome relative to TMB-H2O2 system; (C) SEM image of mAb1-GCE (inset: the pretreated GCE), scale bar: 2 μm; (D) Nyquist diagrams for (a, a0)NHS/EDC-activated GCE and (b, b0)mAb-GCE before(a, b) and after (a0, b0) reaction with target TSH in 10 mmol/L PBS (pH 7.4) containing 10 nmol/L Fe(CN)64-/3- with the range from 10-2 Hz to 105 Hz at an alternate voltage of 5.0 mV. | |
Next, the as-prepared immunosensor was also characterized by using scanning electron microscopy (SEM; Helios G4 CX, FEI, USA) (Fig. 1C). The inset in Fig. 1C gives typical SEM image of the treated GCE. In contrast, the surface became rougher after the pretreated GCE reacted with mAb1 antibodies in the presence of NHS/EDC. To further investigate the conjugation of mAb1 antibody, we utilized electrochemical impedance spectroscopy (EIS) to investigate differently modified electrodes in 10 mmol/L PBS (pH 7.4) containing 10 nmol/L Fe(CN)64-/3- (Fig. 1D). Curves a and b represent the Nyquist diagrams of NHS/EDC-activated GCE and mAb2-modified GCE, respectively. Conjugation of mAb2 caused the increasing resistance of the modified electrode, which was ascribed to the weak conductive biomacromolecules. After that, two corresponding electrodes reacted with the biotinylated pAb2 antibodies, respectively. Almost no shifts in the resistance were observed at the NHS/EDC-activated GCE before and after reaction with pAb2 (curve a0 vs. curve a). A strong increase in the resistance was acquired in the presence of mAb2 (curve b0 vs. curve b), indicating that mAb1 antibody was covalently conjugated to the GCE, which provided a preliminary condition for the immunoassay development.
Typically, antibody or antigen in aqueous solution has a net electrical charge polarity, associated with the isoelectric points of the species and the ionic composition of the solution. Using mAb1-GCE and pAb2-HRPEL, we investigated the feasibility of potentio-metric immunosensor for the detection of 1.0 μIU/mL TSH (as an example) after each step. The electrical potentials were recorded in PBS (10 mmol/L, pH 7.4) (Fig. 2A). As shown from column a, a negative electrical potential of -18.7 mV was gotten at the treated GCE, which might derive from negatively charged hydroxyl or carboxyl groups. Upon activation of NHS/EDC, the electrode potential decreased to -11.9 mV (column b) thanks to the conjugation of uncharged NHS. After conjugation with mAb1, the potential increased to -28.5 mV (column c). Moreover, the electrode potentials gradually increased to -32.7 mV and -46.7 mV after reaction with the TSH (column d) and pAb2-HRPEL (column e), respectively. The reason might be ascribed to the fact that their isoelectric points were less than pH 7.4, which exhibited negative charges in PBS. Significantly, the potential heavily decreased when the released HRP molecules reacted with 4-CN in the presence of H2O2 (column f), indicating the deposited benzo-4-chlorohexadienone could cause the change of potential. To further clarify the amplification efficiency of pAb2-HRPEL, a comparative study was carried out by using pAb2-HRP without the liposome (note: pAb2-HRP was achieved from TSH ELISA kit). As seen from columns f and f', the change in the potential of using pAb2-HRPEL was stronger than that of using pAb2-HRP before and after reaction with 4-CN. Meanwhile, the surface of using pAb2-HRPEL (Fig. 2B) became rougher than that of using pAb2-HRP (Fig. 2C), as shown from SEM images. Therefore, the potentiometric immunosensor could be applied for the detection of target TSH by using enzyme-encapsulated liposome with high sensitivity.

|
Download:
|
| Fig. 2. (A) Electrical potentials of (a, a') the pretreated GCE, (b, b') NHS/EDC-activated GCE, (c, c') mAb1-GCE, (d, d') mAb1-GCE + 1.0 μIU/mL TSH, (e) mAb1-GCE + 1.0 μIU/mL TSH + pAb2-HRPEL, (e') mAb1-GCE + 1.0 μIU/mL TSH + pAb2-HRP, (f) mAb1-GCE + 1.0 μIU/mL TSH + pAb2-HRPEL + 4-CN/H2O2 and (f') mAb1-GCE + 1.0 μIU/mL TSH + pAb2-HRP + 4-CN/H2O2 in PBS (10 mmol/L, pH 7.4); (B, C) SEM images of the modified electrodes by using different signal tags: (B) pAb2-HRPEL and (C) pAb2-HRP. Scale bars in B and C: 2 μm. | |
Under the optimum conditions (immunoreaction time: 35 min; deposition time of 4-CN: 12 min; Fig. S1 in Supporting informa-tion), the prepared mAb1-GCE and pAb2-HRPEL were used for detection of different-level TSH standards. Detailed procedure can be found in Supporting information. As seen from Fig. 3A, the curve was not a linear one, as is commonly observed for immunoassays, and we used curve-fitting for the calibration procedure. A good linear relationship between the electrical potentials and the decimal logarithm of TSH concentrations, however, could be fitted to the experimental points from 0.01 μIU/mL to 30 μIU/mL. The detection limit (LOD) was 0.0067 μIU/mL, as calculated at the 3sblank criterion (n = 11). Obviously, the LOD of our strategy was comparable with existed commercial human TSH ELISA kits from various companies, e.g., Abcam (cat#: ab108659 for 0.05 μIU/mL; cat#: ab108660 for 0.2 μIU/mL) and CusabioⓇ (cat#: CSB-E17548B for 0.225 μIU/mL), and other detection schemes for TSH (Table S1 in Supporting information). Since the concentration of TSH in normal human serum is ≤4.0 μIU/mL, the potentiometric immu-nosensor can meet the requirement of clinical diagnostics.

|
Download:
|
| Fig. 3. (A) Calibration plots of potentiometric immunosensor toward TSH standards with different concentration; (B) The specificity of potentiometric immunosensor against target TSH and nontargets including ACTH, CT, HCG, AFP and CEA. | |
The reproducibility of HRPEL-based potentiometric sensor was investigated by assaying 1.0 μIU/mL TSH (used as an example) with the different mAb1-GCE and pAb2-HRPEL. Experimental results indicated that relative standard deviation (RSD) values were 6.7% and 12.2% (n = 6) for the same-batch and various-batch immuno-sensors and pAb2-HRPEL conjugates, respectively. As expected, the RSD for inter-assay was obviously more than that of intra-assay. Hence, the reproducibility and precision of the potentiometric immunosensor were acceptable.
Fig. 3B shows the specificity of HRPEL-based potentiometric immunosensor against other hormones and biomarkers, e.g., adrenocorticotropic hormone (ACTH), calcitonin (CT), human chorionic gonadotrophin (HCG), alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA). Almost no obvious responses on the potentiometric immunosensor were acquired toward non-target analytes (vs. background signal). Strong signals could be achieved in the presence of target TSH with high selectivity. In addition, the storage (4 C) stability of mAb1-GCE and pAb2-HRPEL was also monitored during a long period. The potentials could preserve 98.4%, 97.2%, 94.5%, 92.1% and 90.7% (n = 3) of the initial signal at the first, second, third, fourth and fifth month, respectively, indicating a good storage stability.
Finally, the accuracy of HRPEL-based potentiometric sensing method was evaluated for the analysis of human serum samples, which were obtained from our hospital according to the rules of the local ethical committee (note: Informed consent was obtained from any experimentation with human subjects.). The obtained results were compared with those of using commercial human TSH ELISA kits from the Clinical Laboratory and Medical Diagnostic Laboratory in our hospital (Table S2 in Supporting information). Evaluation of the accuracy between two methods was executed by using a t-test. Results revealed that all the texp values in all cases were below tcrit (tcrit[2, 0.05] = 4.30), indicating good accuracy between two methods.
In conclusion, this communication successfully constructed a simple and sensitive potentiometric immunosensor for detec-tion of target TSH. HRP-encapsulated nanoliposomes were used for the signal amplification. The immunosensor was prepared using a simple covalent conjugation method. The shift in the potential mainly derived from the deposited benzo-4-chloro-hexadienone on the electrode with an insoluble uncharged organic molecule. Importantly, the conjugated HRPEL after the immunoreaction could be removed using Triton X-100. Signifi-cantly, our strategy can open a new horizon for protein diagnostics and biosecurity.
AcknowledgmentThis work was supported by the Natural Science Foundation of Fujian Province, China (No. 2017J01189).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.06.024.
| [1] |
B. Yang, D. Liu, L. Zhu, et al., Chin. Chem. Lett. 29 (2018) 1879-1882. DOI:10.1016/j.cclet.2018.01.042 |
| [2] |
I. Barakat-Walter, R. Kraftsik, Neural Regeneration Res. 13 (2018) 599-608. DOI:10.4103/1673-5374.230274 |
| [3] |
Z. Yu, Y. Tang, G. Cai, et al., Anal. Chem. 91 (2019) 1222-1226. DOI:10.1021/acs.analchem.8b04635 |
| [4] |
Z. Luo, Q. Qi, L. Zhang, et al., Anal. Chem. 91 (2019) 4149-4156. DOI:10.1021/acs.analchem.8b05959 |
| [5] |
S. Lv, Z. Lin, K. Zhang, et al., Anal. Chim. Acta 964 (2017) 67-73. DOI:10.1016/j.aca.2017.02.004 |
| [6] |
Q. Li, S. Lv, M. Lu, et al., Microchim. Acta 183 (2016) 2815-2822. DOI:10.1007/s00604-016-1929-x |
| [7] |
R. Thurer, T. Vigassy, M. Hirayama, et al., Anal. Chem. 79 (2007) 5107-5110. DOI:10.1021/ac070932m |
| [8] |
M. Kamahori, Y. Ishige, M. Shimoda, Biosens. Bioelectron. 22 (2007) 3080-3085. DOI:10.1016/j.bios.2007.01.011 |
| [9] |
K. Deng, Y. Zhang, X. Tong, Analyst 143 (2018) 1454-1461. DOI:10.1039/C7AN02091K |
| [10] |
N. Silva, J. Magahaes, M. Oliva-Teles, et al., Anal. Methods 7 (2015) 4008-4011. DOI:10.1039/C5AY00053J |
| [11] |
B. Zhang, D. Tang, R. Goryacheva, et al., Chem. -Eur. J. 19 (2013) 2496-2503. DOI:10.1002/chem.201203131 |
| [12] |
B. Zhang, B. Liu, G. Chen, et al., Biosens. Bioelectron. 53 (2014) 465-471. DOI:10.1016/j.bios.2013.10.027 |
| [13] |
K. Zhang, S. Lv, Z. Lin, et al., Biosens. Bioelectron. 101 (2018) 159-166. DOI:10.1016/j.bios.2017.10.031 |
| [14] |
K. Zhang, S. Lv, Z. Lin, et al., Biosens. Bioelectron. 95 (2017) 34-40. DOI:10.1016/j.bios.2017.04.005 |
| [15] |
L. Hou, Y. Tang, M. Xu, et al., Anal. Chem. 86 (2014) 8352-8358. DOI:10.1021/ac501898t |
| [16] |
L. Hou, Z. Gao, M. Xu, et al., Biosens. Bioelectron. 54 (2014) 365-371. DOI:10.1016/j.bios.2013.11.014 |
| [17] |
Z. Luo, L. Zhang, R. Zeng, et al., Anal. Chem. 90 (2018) 9568-9575. DOI:10.1021/acs.analchem.8b02421 |
| [18] |
Y. Lin, Q. Zhou, D. Tang, Anal. Chem. 89 (2017) 11803-11810. DOI:10.1021/acs.analchem.7b03451 |
| [19] |
Y. Lin, Q. Zhou, Y. Zeng, et al., Microchim. Acta 185 (2018) 311. DOI:10.1007/s00604-018-2848-9 |
| [20] |
J. Tang, Y. Huang, H. Liu, et al., Biosens. Bioelectron. 79 (2016) 508-514. DOI:10.1016/j.bios.2015.12.097 |
| [21] |
J. Israelachvili, D. Mitechell, Biochim. Biophys. Acta 389 (1975) 13-29. DOI:10.1016/0005-2736(75)90381-8 |
| [22] |
K. Edwards, A. Baeumner, Anal. Chem. 79 (2007) 1806-1815. DOI:10.1021/ac061471s |
 2020, Vol. 31
2020, Vol. 31 

