Plant diseases have long been recognized as a serious threat to global agricultural production. Among them, phytopathogenic bacteria and fungi are difficult to control and cause enormous crop yield losses per year [1, 2]. For instance, Xanthomonas oryzae pv. oryzae (Xoo) is a pathogen responsible for rice bacterial leaf blight, which can reduce rice yield by up to 50% under certain conditions [3]. On the other hand, it is well-known that fungal diseases remain the biggest threat to global agricultural industry at all times [2]. Compared with other methods, chemical control has some unique advantages, including high effectiveness, low cost and convenient application. However, some severe problems had emerged due to long-term unreasonable utilization of the existing antimicrobial agents, such as growing germicide resistances, decreasing control effects and environmental pollution [4, 5]. Therefore, the search of new and more efficient agricultural germicides is still an urgent task in the field of crop protection.
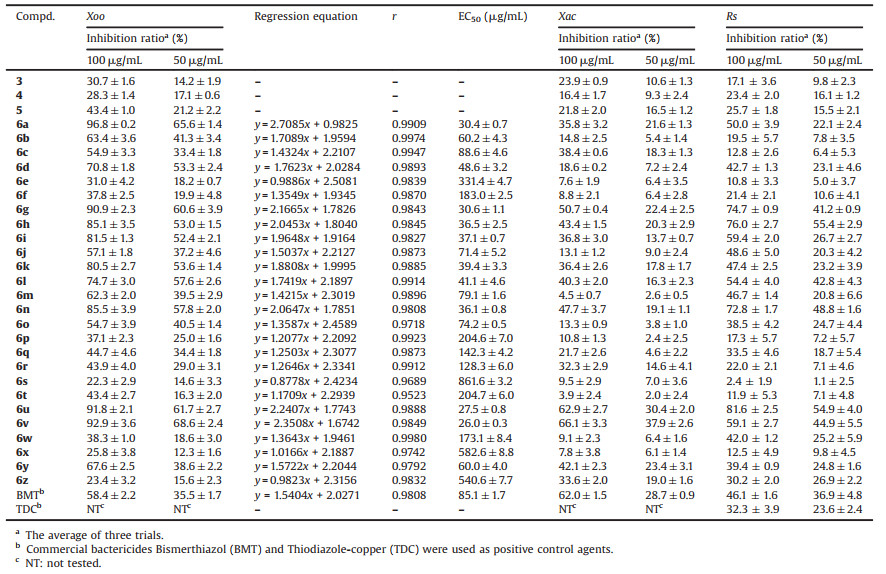
Previous studies had shown that 1, 3, 4-oxadiazole thioether derivatives possessed a wide range of pesticidal activities, including antibacterial [6-8], nematicidal [9], antifungal [10], and anti-TMV activities [11]. Generally speaking, the presence of thioether group in bioactive molecules was believed to increase the hydrophilicity and provide an additional hydrogen-bond accepting site for ligand-protein binding, which were favorable for improving the pesticide-likeness [12, 13]. On the other hand, quinazoline ring was one of the most significant nitrogencontaining heterocycles, occupying an important position in medicine and pesticide chemistry [14]. Some quinazoline-based drugs and pesticides have been on the market for many years, like anticancer drugs Gefitinib and Vandetanib, acaricide Fenazaquin and sympatholytic drug Prazosin (Fig. 1). As is known, the method of pharmacophore hybridization [15, 16] is quite practical for the discovery of new drugs/pesticides as it can provide multiple interaction sites within a single molecule to interact with relevant proteins of pathogenic microorganisms. Tiodazosin (Fig. 1), a potent competitive postsynaptic α-adrenergic receptor antagonist, is just an excellent example of the delicate combination of 1, 3, 4-oxadiazole thioether group and quinazoline backbone via a piperazinyl carbonyl linkage.
|
Download:
|
| Fig. 1. Some representative bioactive molecules containing either the 1, 3, 4-oxadiazole thioether group or a quinazoline moiety. | |
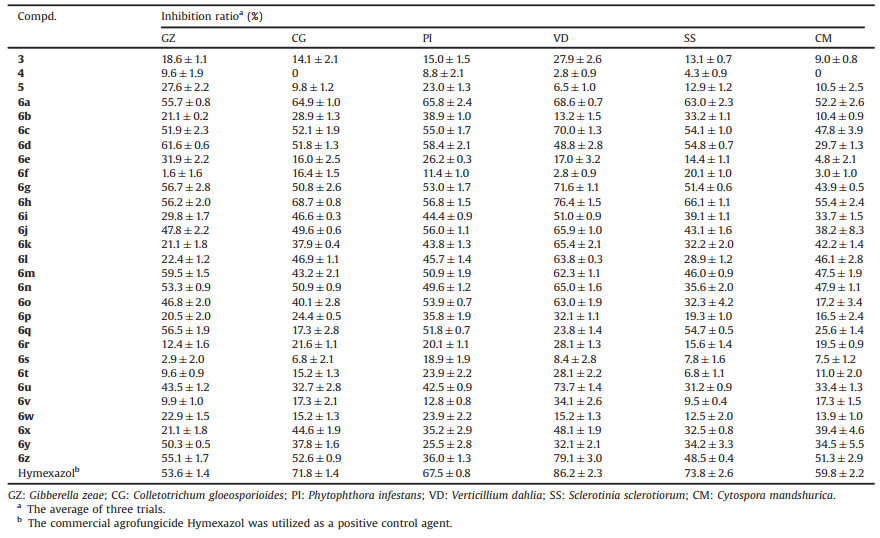
In our previous work [4], a series of 1, 2, 4-triazole thioether derivatives containing both quinazolinylpiperidinyl moiety and acetamide group were synthesized and some compounds exhibited better antibacterial activities against Xoo in vitro, relative to control agent Bismerthiazol. Unfortunately, nearly all the compounds failed to display notable fungicidal activity against tested phytopathogenic fungi at 50 μg/mL. In fact, antimicrobial capabilities of this class of compounds were less satisfied on the whole. As we all know, the introduction of fluoro group into bioactive molecules can improve the lipophilicity, bioavailability and metabolic stability [17-21]. Based on the aforementioned considerations as well as our prior work [4], we thus designed a series of 1, 3, 4-oxadiazole thioether derivatives containing the 6-fluoroquinazolinylpiperidinyl moiety using pharmacophore hybridization method (with the capacity to overcome the resistance, reducing the toxicity and improve the pharmacokinetic properties) [22] and also took account of the following points: (a) replacing the 1, 2, 4-triazole ring with its bioisosteric 1, 3, 4-oxadiazole ring; (b) introducing a fluoro group into the quinazoline backbone; (c) simplifying the final structure by removing the amide group (Fig. 2).

|
Download:
|
| Fig. 2. Design strategy of target compounds in this work. | |
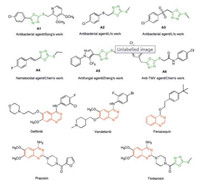
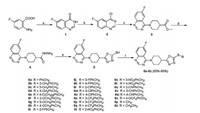
Synthetic procedures for target compounds 6a–6z were depicted in Scheme 1. First, 4-chloro-6-fluoroquinazoline 2 [23] was reacted with methyl 4-piperidinecarboxylate in 1, 4-dioxane solution to afford ester 3, which was then subjected to hydrazinolysis for the preparation of hydrazide 4. Next, hydrazide 4 was treated with CS2 under basic conditions and then acidified to furnish oxadiazole-thiol 5. Finally, target compounds 6a–6z were readily obtained in 55%–95% yield by reaction of thiol 5 with various halogenated hydrocarbons in K2CO3/CH3CN system at room temperature. The structures of target compounds 6a–6z were fully characterized by 1H NMR, 13C NMR and HRMS spectra. Detailed synthetic procedures, characterization data and spectral copies of intermediates 3–5 and target compounds 6a–6z are available in Supporting information. Fortunately, a single crystal of compound 6d suitable for X-ray diffraction analysis (Fig. 3) was obtained by slow evaporation of a DMF solution of 6d at room temperature.
|
Download:
|
| Scheme 1. Synthesis of target compounds 6a–6z. Reagents and conditions: (a) HCONH2, reflux, 7 h; (b) SOCl2, DMF, reflux, 5 h; (c) methyl 4-piperidinecarboxylate, 1, 4-dioxane, reflux, 7 h; (d) hydrazine hydrate, MeOH, r.t., overnight; (e) ⅰ) NaOH, EtOH, CS2, r.t., 15 min then reflux 8 h; ⅱ) 10% HCl, pH 5.5; (f) RX, K2CO3, CH3CN, r.t., 8–12 h. | |
|
Download:
|
| Fig. 3. Crystal structure of compound 6d (CCDC: 1913061). | |
In vitro inhibition activities of target compounds 6a-6z and intermediates 3–5 against three pathogenic bacteria Xoo, Rs (Ralstonia solanacearum) and Xac (Xanthomonas axonopodis pv. citri) were assessed based on the turbidimetric method [4, 24], with commercially-available agrobactericides Bismerthiazol (BMT) and Thiodiazole-copper (TDC) as positive control agents. As listed in Table 1, some of the target compounds demonstrated significantly better antibacterial efficacy towards the pathogens under the tested concentrations, relative to control agents. For example, compounds 6a, 6g, 6h, 6n, 6u and 6v showed the inhibition ratios of 96.8%, 90.9%, 85.1%, 85.5%, 91.8% and 92.9% against Xoo at 100 μg/mL, respectively, which were considerably superior to that of control BMT (58.4%). Unfortunately, only two compounds within the series (namely compounds 6u and 6v) were found to possess a comparable inhibition activity against Xac at 100 μg/mL, in comparison to control BMT (62.0%). As far as the bacterium Rs was concerned, compounds 6g, 6h, 6n and 6u had more potent inhibition efficacies of 74.7%, 76.0%, 72.8% and 81.6% at 100 μg/mL, respectively, compared with control agents BMT (46.1%) and TDC (32.3%).
|
|
Table 1 Antibacterial activities of target compounds 6a-6z and intermediates 3, 4 as well as 5 against phytopathogenic bacteria Xoo, Xac and Rs. |
In consideration of outstanding antibacterial activites exhibited by most of the target compounds against Xoo, EC50 values (half-maximal effective concentration) of all the target compounds were further determined. As summarized in Table 1, more than half of the target compounds had a clearly lower EC50 value than control BMT. It was noteworthy that compounds 6a, 6g, 6h, 6i, 6k, 6l, 6n, 6u and 6v possessed EC50 values of 30.4, 30.6, 36.5, 37.1, 39.4, 41.1, 36.1, 27.5 and 26.0 μg/mL, respectively, over two times more potent than control BMT (85.1 μg/mL). A preliminary structure-activity relationship analysis demonstrated the following findings: (a) The introduction of strong electron-withdrawing groups in the benzene ring of benzyl group was detrimental to antibacterial activity, like -CF3, -NO2 and -OCF3 groups; (b) The presence of a cyano group at the 2-or 3-position of the benzyl group (compounds 6u and 6v) was found to be most beneficial to bactericidal activity against Xoo, which may be attributed to the formation of an additional hydrogen bond between cyano group (being a strong hydrogen-bond acceptor) and relevant bacterial protein [25]; (c) The presence of a mono-halogen substituent (such as -F and -Cl) on the phenyl ring was helpful to antibacterial activity on the whole, in comparison with other substituents; (d) The existence of sterically bulky tert-butyl group (6e), strong electron-withdrawing -OCF3 (6x) or 3-NO2 (6s) groups or ethyl thioether unit (6z) in the target compounds led to the weakest antibacterial effect.
In vitro antifungal activities of target compounds 6a–6z and intermediates 3–5 were also evaluated using the mycelial growth rate method [4, 5], against six types of phytopathogenic fungi. As shown in Table 2, some of the target compounds displayed a pronounced fungicidal effect against certain fungi at 50 μg/mL. For example, compounds 6a, 6c, 6d, 6g, 6h, 6j, 6m, 6n, 6q, 6y and 6z exhibited a significant antifungal activity towards G. zeae, slightly higher or similar to that of control Hymexazol (53.6%). Concerning the fungus C. gloeosporioides, only compounds 6a and 6h were found to exhibit comparable antifungal activities to control Hymexazol (71.8%). It should be noted that seven compounds (including 6a, 6c, 6g, 6h, 6m, 6n and 6z) possessed broad-spectrum fungicidal activities against all six fungi, having an inhibition ratio of >50% in nearly every case.
|
|
Table 2 Antifungal activities of target compounds 6a–6z and intermediates 3, 4 and 5 at 50 μg/mL. |
In summary, a series of novel 6-fluoroquinazolinylpiperidinyl-containig 1, 3, 4-oxadiazole thioether derivatives were evaluated as agricultural antimicrobial agents. In vitro antibacterial bioassays indicated that compounds 6a, 6g, 6u and 6v had significantly higher inhibiton activity against phytopathogenic bacterium Xoo, relative to commercially-available bactericide Bismerthiazol. Additionally, in vitro antifungal assays showed that seven compounds demonstrated broad-spectrum fungicidal activities against tested phytopathogenic fungi at 50 μg/mL. The present work demonstrated the potential of fluoroquinazolinylpiperidinylcontaining 1, 3, 4-oxadiazole thioether derivatives as effective antimicrobial agents for crop protection, deserving more considerations in future studies.
AcknowledgmentsThis work was financially supported by Young Top-Notch Talent Support Program of Guizhou Provincial Education Department (No. 2018038), Guizhou Provincial High-Level Overseas Talents Innovation and Enterpreneurship Program (No. 201809) and Breeding Program of Guizhou University (No. 20185781).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.06.037.
| [1] |
F.P. Carvalho, Environ. Sci. Policy 9 (2006) 685-692. DOI:10.1016/j.envsci.2006.08.002 |
| [2] |
L.L. Wang, C. Li, Y.Y. Zhang, C.H. Qiao, Y.H. Ye, J. Agric. Food Chem. 61 (2013) 8632-8640. DOI:10.1021/jf402388x |
| [3] |
F.D. Lorenzo, A. Palmigiano, A. Silipo, et al., Carbohydr. Res. 427 (2016) 38-43. DOI:10.1016/j.carres.2016.03.026 |
| [4] |
L. Yang, X.P. Bao, RSC Adv. 7 (2017) 34005-34011. DOI:10.1039/C7RA04819J |
| [5] |
M. Zhang, Z.C. Dai, S.S. Qian, et al., J. Agric. Food Chem. 62 (2014) 9637-9643. DOI:10.1021/jf504359p |
| [6] |
X.P. Song, P. Li, M.W. Li, et al., Pestic. Biochem. Physiol. 147 (2018) 11-19. DOI:10.1016/j.pestbp.2017.10.011 |
| [7] |
P. Li, P.Y. Tian, Y.Z. Chen, et al., Pest Manag. Sci. 74 (2018) 2844-2852. |
| [8] |
P. Li, D.Y. Hu, D.D. Xie, et al., J. Agric. Food Chem. 66 (2018) 3093-3100. DOI:10.1021/acs.jafc.7b06061 |
| [9] |
J.X. Chen, Y.Z. Chen, X.H. Gan, et al., J. Agric. Food Chem. 66 (2018) 9616-9623. DOI:10.1021/acs.jafc.8b03020 |
| [10] |
Y.T. Zheng, T.T. Zhang, P.Y. Wang, et al., Chin. Chem. Lett. 28 (2017) 253-256. DOI:10.1016/j.cclet.2016.06.055 |
| [11] |
L. Chen, P.Y. Wang, Z.X. Li, et al., Chin. J. Chem. 34 (2016) 1236-1244. DOI:10.1002/cjoc.201600501 |
| [12] |
Y.H. Li, B. Zhang, H.K. Yang, et al., Eur. J. Med. Chem. 125 (2017) 1098-1106. DOI:10.1016/j.ejmech.2016.10.051 |
| [13] |
A.V. Bogolubsky, Y.S. Moroz, P.K. Mykhailiuk, et al., ACS Comb. Sci. 17 (2015) 348-354. DOI:10.1021/acscombsci.5b00024 |
| [14] |
I. Khan, A. Ibrar, N. Abbas, A. Saeed, Eur. J. Med. Chem. 76 (2014) 193-244. DOI:10.1016/j.ejmech.2014.02.005 |
| [15] |
L. Krstulović, I. Stolić, M. Jukić, et al., Eur. J. Med. Chem. 137 (2017) 196-210. DOI:10.1016/j.ejmech.2017.05.054 |
| [16] |
D. Havrylyuk, O. Roman, R. Lesyk, Eur. J. Med. Chem. 113 (2016) 145-166. DOI:10.1016/j.ejmech.2016.02.030 |
| [17] |
J. Wang, M. Sánchez-Roselló, J.L. Aceña, et al., Chem. Rev. 114 (2014) 2432-2506. DOI:10.1021/cr4002879 |
| [18] |
E.P. Gillis, K.J. Eastman, M.D. Hill, D.J. Donnelly, N.A. Meanwell, J. Med. Chem. 58 (2015) 8315-8359. DOI:10.1021/acs.jmedchem.5b00258 |
| [19] |
S. Zhou, S. Zhou, Y.T. Xie, et al., Chin. Chem. Lett. 29 (2018) 1254-1256. DOI:10.1016/j.cclet.2017.10.022 |
| [20] |
C.C. Wu, B.L. Wang, J.B. Liu, et al., Chin. Chem. Lett. 28 (2017) 1248-1251. DOI:10.1016/j.cclet.2017.01.019 |
| [21] |
L.Y. Zhang, B.L. Wang, Y.Z. Zhan, et al., Chin. Chem. Lett. 27 (2016) 163-167. DOI:10.1016/j.cclet.2015.09.015 |
| [22] |
F. Gao, T. Wang, J. Xiao, G. Huang, Eur. J. Med. Chem. 173 (2019) 274-281. DOI:10.1016/j.ejmech.2019.04.043 |
| [23] |
P.Q. Zhang, B.A. Song, S. Yang, et al., Chin. J. Org. Chem. 26 (2006) 1275-1278. |
| [24] |
X. Wang, J. Yin, L. Shi, G.P. Zhang, B.A. Song, Eur. J. Med. Chem. 77 (2014) 65-74. DOI:10.1016/j.ejmech.2014.02.053 |
| [25] |
N. Mast, W. Zheng, C.D. Stout, I.A. Pikuleva, J. Biol. Chem. 288 (2013) 4613-4624. DOI:10.1074/jbc.M112.438754 |
 2020, Vol. 31
2020, Vol. 31