b State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China
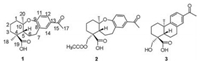
Callicarpa bodinieri, belonging to the genus of Callicarpa in Verbenaceae family [1], has been widely used in traditional medicine for the treatment of inflammation [2], rheumatism, hematemesis, and gastrointestinal bleeding [3]. Previous phytochemical investigations of the leaves and twigs of C. bodinieri has led to the identification of flavonoids [4], lignans [5-7], glycosides [8-10], phenolic acids [11, 12], triterpenoids [13, 14], and diterpenoids [15-20], etc. In this study, two new 9, 10-seco and etherified abietane-type diterpenoids bodinieric acids J and K (1 and 2) and one known compound (3) were isolated and identified from Callicarpa bodinieri (Fig. 1). In order to clarify the biological source of 9, 10-seco and etherified abietane-type scaffold, the biosynthetic pathways of compounds 1-3 were preliminarily elucidated. Based on the medicinal utilization of this plant for the treatment of immunity-related diseases, inhibitory activities against NLRP3 inflammasome activation of 1-3 were evaluated.
|
Download:
|
| Fig. 1. Structure of compounds 1-3 isolated from Callicarpa bodinieri. | |
Stereochemistry is a critical structural element for the presented physical and biological properties of an organic molecule. However, the incorrectly assigned structures are not rare, even with many new experimental techniques and methods emerged nowadays. DP4+ is one of the most sophisticated approaches that has been applied to the challenging task of stereo-chemical assignment using the probabilities from the differences between DFT-GIAO calculated and experimental NMR data [21, 22]. Along with the extensive application of DP4+ in NMR calculations last few years [23], it has been becoming an increasingly popular method for the structure elucidation in organic and natural product chemistry.
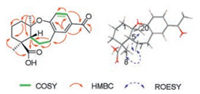
Bodinieric acid J (1) was isolated as a white powder; [α]D22 -3.2 (c 0.28, MeOH). Its molecular formula was deduced to be C19H24O4 from the sodium adduct molecular ion at m/z 339.1571 in the positive-ion HRESIMS spectrum. The IR spectrum exhibited absorptionbands at3386, 1740, 1672, 1595, 1279, 1133, 1042 cm-1 (as shown in Fig. S8 in Supporting information), which reminded the presence of hydroxy, carboxyl, ether and aryl groups. These NMR data listed in Table 1 revealed that compound 1 was closely related to the abietane-type diterpenoid 3 that previously isolated from this plant by our group [24]. According to 1H–1H COSY spectrum, the key correlations of H-5/H2-6/H2-7 and H-11/H-12 were shown in Fig. 2. HMBC correlations of H2-1 with C-2 and C-3;H-5 with C-6 and C-19; H2-7 with C-5 and C-6;H3-18 with C-3 and C-19; H3-20 with C-1, C-5 and C-10; H-14 with C-7 and C-9 revealed the presence of a routine A-ring and part of B-ring structure in abietane-skeleton. The H-12 with C-11 and C-15; H-14 with C-12 and C-15; H3-17 with C-15 correlations undoubtedly established its C-ring and isopropyl unit. In addition, C-9 and C-10 in B-ring were speculated to be disconnected and bridged by an oxygen atom according to the downfield 13C NMR chemical shift at δC 160.3 (C-9) and 85.0 (C-10), as well as its molecular formula. Thus, the planar structure of 1 was confirmed.
|
|
Table 1 1H (600 MHz) and 13C (150 MHz) NMR data of compounds 1 and 2 (δ in ppm, J in Hz, acetone-d6). |
|
Download:
|
| Fig. 2. Key 1H-1H COSY, HMBC, and ROESY correlations of 1. | |
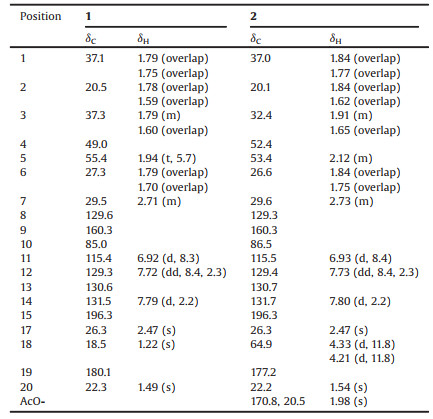
The relative configuration of 1 was preliminarily inferred from a ROESY experiment, which showed correlations between: H3-20 and H-7α; H-7α and H3-18 (Fig. 2). The configuration of 1 was further elucidated by the comparison of experimental and calculated NMR spectrum data. The NMR data of four different isomers that 4S, 5S, 10R (isomer 1), 4S, 5S, 10S (isomer 2), 4S, 5R, 10R (isomer 3) and 4S, 5R, 10S (isomer 4) were calculated using GIAO method [25]. The comparison of the computed results and experimental data were then processed by DP4+ probability statistical analysis [26, 27]. Details of the computational procedure for NMR calculation can be found in Supporting information. As shown in Table 2, the isomer of 4S, 5R, 10R was 100% consistent with experimental NMR data. Thus, the structure of 1 was consequently established and named as bodinieric acid J.
|
|
Table 2 Comparison of the computed results and experimental data of compound 1 processed by DP4+ statistical analysis. |
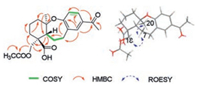
Bodinieric acid K (2) was purified as white microcrystalline solid, with [α]D22 -14.9 (c 0.60, MeOH). Its positive ion HRESIMS revealed a peak for a sodium adduct ion at m/z 397.1622, corresponding to a molecular formula of C21H26O6Na. Compared the 1H and 13C NMR data (Table 1) with that of compound 1, their only difference was the additional presence of a CH3CO-group that bonded with C-18 hydroxyl to generate an ester in 2. This assignment could be confirmed by the HMBC correlations of H2-18 with CH3CO-, H2-3 and C-19; H-5 with C-18 and C-19 (Fig. 3). The ROESY correlations were also similar to those of 1. Based on the biosynthetic considerations of natural products, the configuration of 2 was speculated to stay the same way. Consequently, the structure of 2 was then confirmed and named as bodinieric acid K.
|
Download:
|
| Fig. 3. Key 1H-1H COSY, HMBC, and ROESY correlations of 2. | |
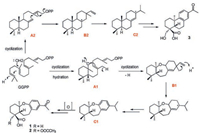
To better understand the biosynthesis mechanism of 9, 10-seco and etherified B-ring of abietane-type scaffold, the biosynthetic pathways of compounds 1-3 were preliminarily elucidated as shown in Fig. 4. Compared with the synthesis of common abietaneskeleton in 3, the variant core structure of B-ring in compounds 1 and 2 were derived initially from the hydratization of 5, 10-alkenyl in GGPP to form intermediate A1. 9, 10-seco and etherified fragment in B1 was further generated by the cycloaddition of 10-hydroxyl and 8, 9-alkenyl. Herein, the unusual abietane-type diterpene of C1 was finally obtained through methyl rearrangement and dehydrogenation reactions.
|
Download:
|
| Fig. 4. Hypothetical biosynthetic pathways of compounds 1-3. | |
The leaves and twigs of Callicarpa bodinieri have been used as traditional Chinese medicine for thousands of years in the treatment of inflammation-related diseases, especially pyroptosis-related diseases, such as rheumatoid arthritis and gout [28]. Inflammasome plays a key role in autoinflammatory and autoimmune diseases, such as gout, rheumatoid arthritis etc. [29]. Its activation causes an inflammatory form of programmed cell death of macrophages called pyroptosis, which leads to maturation of Caspase-1 and release of inflammatory mediators, including IL-1β and IL-18. Excessive IL-1β production is harmful and causes immune dysfunctions [30]. Thus, the isolated compounds were firstly evaluated for the inhibition of pyroptosis, which was induced by NLRP3 inflammasome activation.
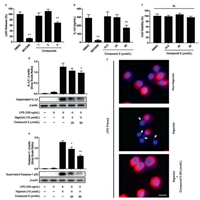
The effects of compound 1-3 on pyroptosis inhibition were tested in mouse macrophage J774A.1 cell. Among the three compounds, compound 3 inhibited LDH (Lactate dehydrogenase) release in J774A.1 cell rather than compounds 1 and 2 (Fig. 5a). To furtherly detect whether compound 3 inhibits inflammasome activation, the IL-1β secretion and maturation as well as Caspase-1 maturation were evaluated. As shown in Fig. 5b, compound 3 significantly inhibited IL-1β secretion. Besides, MTT assay showed that cell viabilities were not affected by compound 3 at concentrations 12.5 -50 μmol/L (Fig. 5c), suggesting that the effects of compound 3 on J774A.1 cell were not attributable to its cytotoxicity. Furthermore, compound 3 blocked IL-1β and Caspase-1 maturation in Western blot assays (Figs. 5d and e). Compound 3 also inhibited ASC (apoptosis-associated speck-like protein containing a CARD) specks formation in macrophages (Fig. 5f). These results suggest that compound 3 inhibits LPS and Nigericin induced NLRP3 inflammasome activation in mouse macrophages.
|
Download:
|
| Fig. 5. Compounds from Callicarpa bodinieri inhibits NLRP3 inflammasome activation. (a) LDH release assay. MCC950 was used as a positive control. (b) Supernatant IL-1β inhibition. (c) Cytotoxicity of compound 3. (d, e) Western blot assays for supernatant IL-1β and Caspase-1 treated by compound 3. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01. ns, not significant. (f) ASC formation treated by compound 3. Blue = DAPI, red = ASC, white arrows indicated ASC specks, scale bar = 20 μm. | |
In summary, two new 9, 10-seco and etherified abietane diterpenoids bodinieric acids J and K (1 and 2) and one known compound (3) were isolated from the leaves and twigs of Callicarpa bodinieri. Their chemical structures were elucidated by detailed spectrometry data analysis as well as DP4+ NMR calculation. Hypothetical biosynthetic pathways of 1-3 were also preliminarily speculated. Compound 3 inhibited macrophage pyroptosis and exhibited blockage of NLRP3 inflammasome activation in vitro.
AcknowledgmentsThis project was financially supported by the National Natural Science Foundation of China (Nos. 81422046 and 21762048), the Yunnan Applicative and Basic Research Program (Nos. 2015BC002, 2018FY001 and 2018FA048), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT_17R94), the Key Program of Natural Science of Yunnan Province to W.L. Xiao, the Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education (No. 2017KF02), the Natural Science Foundation of Yunnan University (No. 2017YDQN03) and the Foundation of Yunnan Educational Committee (No. 2018JS002). We thank the high-performance computing center of Yunnan University and KIB (CAS) for providing the computational resources.
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.09.020.
| [1] |
F.Z. Ren, G.Y. Niu, X.H. Luan, et al., Nat. Prod. Res. Dev. 15 (2003) 155-156. |
| [2] |
F. Ren, H. Qu, X. Luan, et al., Nat. Prod. Res. Dev. 13 (2001) 33-34. |
| [3] |
J. Song, The Study on Evaluation of in vitro Coagulation Effects and Antibacterial of Chinese Herbals, Master's Degree Thesis, Guangxi University, 2016.
|
| [4] |
J.J. Liang, J.L. Qi, L. Li, et al., Chem. Nat. Compd. 47 (2011) 110-111. DOI:10.1007/s10600-011-9846-z |
| [5] |
Y. Shao, L.H. Hu, K.Y. Sim, et al., Helv. Chim. Acta 89 (2006) 64-72. DOI:10.1002/hlca.200690013 |
| [6] |
M. Ono, K. Mishima, T. Yamasaki, et al., J. Nat. Med. 63 (2009) 86-90. DOI:10.1007/s11418-008-0289-1 |
| [7] |
Y.H. Luo, Z.Q. Zhou, S.C. Ma, et al., Phytochem. Lett. 7 (2014) 194-197. DOI:10.1016/j.phytol.2013.12.001 |
| [8] |
A.Z. Wu, Y.J. Zhai, Z.X. Zhao, et al., Fitoterapia 84 (2013) 237-241. DOI:10.1016/j.fitote.2012.12.014 |
| [9] |
D.A. Dias, C.A. Silva, S. Urban, Planta Med. 75 (2009) 1442-1447. DOI:10.1055/s-0029-1185724 |
| [10] |
T. Yamasaki, C. Masuoka, T. Nohara, et al., J. Nat. Med. 61 (2007) 318-322. DOI:10.1007/s11418-007-0135-x |
| [11] |
J.Q. Yuan, L. Qiu, L.H. Zou, et al., Helv. Chim. Acta 98 (2015) 482-489. DOI:10.1002/hlca.201400206 |
| [12] |
E.L. Xie, G.P. Zhou, T.F. Ji, et al., Chin. Chem. Lett. 20 (2009) 827-829. DOI:10.1016/j.cclet.2009.03.022 |
| [13] |
G.P. Zhou, Y. Yu, M.M. Yuan, et al., Molecules 20 (2015) 9071-9083. DOI:10.3390/molecules20059071 |
| [14] |
M.M. Yuan, R.J. Zhong, G. Chen, et al., J. Asian Nat. Prod. Res. 17 (2015) 138-142. DOI:10.1080/10286020.2014.969246 |
| [15] |
Z.H. Wang, C. Niu, D.J. Zhou, et al., Molecules 22 (2017) 842-848. DOI:10.3390/molecules22050842 |
| [16] |
J. Xu, Y.H. Sun, M.C. Wang, et al., J. Nat. Prod. 78 (2015) 1563-1569. DOI:10.1021/acs.jnatprod.5b00018 |
| [17] |
L. Zhang, M.S. Liu, J. Huang, et al., J. Asian Nat. Prod. Res. 16 (2014) 216-221. DOI:10.1080/10286020.2013.841675 |
| [18] |
L. Zhang, L. Dong, J. Huang, et al., Fitoterapia 89 (2013) 218-223. DOI:10.1016/j.fitote.2013.05.022 |
| [19] |
C.Z. Lin, C.C. Zhu, Z.X. Zhao, et al., Fitoterapia 83 (2012) 1-5. DOI:10.1016/j.fitote.2011.09.013 |
| [20] |
J.J. Chen, H.M. Wu, C.F. Peng, et al., Planta Med. 75 (2009) 1076-1076. |
| [21] |
K. Ermanis, K.E.B. Parkes, T. Agback, et al., Org. Biomol. Chem. 15 (2017) 8998-9007. DOI:10.1039/C7OB01379E |
| [22] |
N. Grimblat, M.M. Zanardi, A.M. Sarotti, J. Org. Chem. 80 (2015) 12526-12534. DOI:10.1021/acs.joc.5b02396 |
| [23] |
M.M. Zanardi, F.A. Biglione, M.A. Sortino, et al., J. Org. Chem. 83 (2018) 11839-11849. DOI:10.1021/acs.joc.8b01749 |
| [24] |
J.B. Gao, S.J. Yang, Z.R. Yan, et al., J. Nat. Prod. 81 (2018) 998-1006. DOI:10.1021/acs.jnatprod.7b01082 |
| [25] |
M.W. Lodewyk, M.R. Siebert, D.J. Tantillo, Chem. Rev. 112 (2012) 1839-1862. DOI:10.1021/cr200106v |
| [26] |
S.G. Smith, J.M. Goodman, J. Am. Chem. Soc. 132 (2010) 12946-12959. DOI:10.1021/ja105035r |
| [27] |
N. Grimblat, M.M. Zanardi, A.M. Sarotti, J. Org. Chem. 80 (2015) 12526-12534. DOI:10.1021/acs.joc.5b02396 |
| [28] |
L. Zhang, J. Huang, M.S. Liu, et al., Heterocycles 87 (2013) 1561-1569. DOI:10.3987/COM-13-12738 |
| [29] |
M.S.J. Mangan, E.J. Olhava, W.R. Roush, et al., Nat. Rev. Drug Discov. 17 (2018) 588-606. DOI:10.1038/nrd.2018.97 |
| [30] |
L.A. Joosten, M.M. Helsen, T. Saxne, et al., J. Immuno. 163 (1999) 5049-5055. DOI:10.1084/jem.190.9.1357 |
 2020, Vol. 31
2020, Vol. 31