b School of Pharmacy, Southwest Medical University, Luzhou 646000, China;
c Qinghai Provincial Key Laboratory of Tibetan Medicine Research and Key Laboratory of Tibetan Medicine Research, Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Xining 810008, China;
d School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, China
Transition-metal-mediated selective cleavage of C—C bond has been regarded as an intriguing and powerful protocol for the construction of various complex organic molecules [1]. In the past decades, many strategies involving C—C bond cleavage have been significantly developed, such as ring-opening of strained molecules with three-or four-membered rings [2], decarbonylation of ynones [3], C—C bond cleavage of ketones [4]. Despite the great progress that has been made in this field, the development of new C—C bond cleavage method to access important valuable organic compounds is still highly desirable.
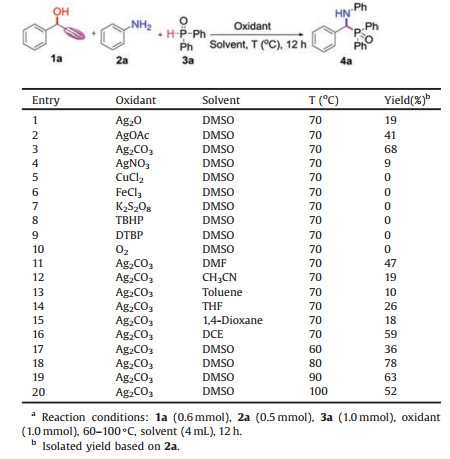
Propargyl alcohols as readily available building blocks have been widely used in organic synthesis [5]. Generally, the reactions using propargyl alcohols mainly focus on the propargylic substitutions [6] and rearrangements [7]. So far, only a few examples of C—C bond cleavage reactions of propargyl alcohols have been reported owing to the relative inertness and thermodynamic stability of C—C bond [8]. In this context, propargyl alcohols were usually utilized as alkynylation reagents to construct the alkynylation products via deacetonative coupling reactions catalyzed by Pd and Rh salts in high temperature [9]. For example, Wu and co-workers reported palladium-catalyzed oxidative deacetonative coupling of tert-propargyl alcohols with H-phosphonates leading to alkynylphosphonates through C—C cleavage process (Scheme 1a) [9c]. Multicomponent reactions as one of the most versatile and powerful synthetic protocols have attracted an increasing attention to assemble complex compounds from simple and readily available starting materials [10]. As our ongoing interest in the synthesis of organic phosphorus compounds [11] and multicomponent reactions [12], herein, we wish to report a new and efficient silver mediated aminophosphinoylation of propargyl alcohols with amines and H-phosphine oxides leading to α-aminophosphine oxides, which are an important class of organic phosphorus compounds that exhibit a wide range of biological and pharmacological activities [13]. The present methodology provides a convenient and alternative approach to access various α-aminophosphine oxides with moderate to good yields, in which the new dealkynylation coupling was achieved via C—C bond cleavage of propargyl alcohols (Scheme 1b).
|
Download:
|
| Scheme 1. Synthetic methods for synthesis of organic phosphorus compounds via C—C bond cleavage of propargyl alcohols. | |
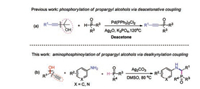
Initially, 1-phenylprop-2-yn-1-ol (1a), aniline (2a) and diphenylphosphine oxide (3a) were chosen as model substrates to optmize the reaction parameters. When the model reaction was performed in DMSO at 70 ℃ under air in the presence of Ag2O (2 equiv.), the product 4a was isolated in 19% yield (Table 1, entry 1). Next, various oxidants were investigated to improve the reaction efficiency (Table 1, entries 2–10). To our delight, the yield of product 4a was increased to 68% when Ag2CO3 was employed as oxidant (Table 1, entry 3). When other oxidants such as CuCl2, FeCl3, K2S2O8, TBHP, DTBP, and O2 were examined, none of desired product 4a was detected (Table 1, entries 5–10). The screening of solvents indicated that DMSO was still optimal than others such as DMF, CH3CN, Toluene, THF, 1, 4-dioxane and DCE (Table 1, entries 3, 11–16). Further investigation of the reaction temperature showed the highest yield of 4a (78%) was obtained when the reaction was conducted at 80 ℃ (Table 1, entry 18). The decrease or increase of reaction temperature would lead to the lower reaction efficiency (Table 1, entries 17, 19 and 20).
|
|
Table 1 Screening of the reaction conditions.a |
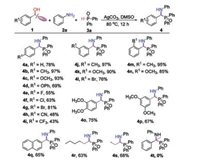
With the optimal condtions in hand, the scope of propargyl alcohols was firstly examined and the results were shown in Scheme 2. In general, various aryl propargyl alcohols containing para-substituted electron-donating or electron-withdrawing groups were compatible for this transformation, giving corresponding products 4a-4i in moderate to excellent yields. Aryl propargyl alcohols bearing electron-donating groups were converted to the corresponding compounds 4b-4d in higher yields relative to the electron-withdrawing substituted analogs (4e-4i). meta-or ortho-substituted aryl propargyl alcohols were also well tolerated in the reaction, affording the desired products 4j-4n in 76%–97% yields. Additionally, multi-substituted aryl propargyl alcohols reacted smoothly with aniline and diphenylphosphine oxide, giving the corresponding products 4o and 4p in 75% and 67% yields, respectively. 1-(Naphthalen-2-yl)prop-2-yn-1-ol could also afford 4q in 65% yield. It should be noted that aliphatic propargylic alcohols were also suitable substrates leading to the products 4r and 4s in good yields. Nevertheless, when 2-phenylbut-3-yn-2-ol was used in the present reaction system, none of the desired prodcut 4t was observed.
|
Download:
|
| Scheme 2. Substrate scope of propargylic alcohols. Reaction conditions: 1 (0.6 mmol), 2a (0.5 mmol), 3a (1.0 mmol), Ag2CO3 (1.0 mmol), DMSO (4 mL), 80 ℃, 12 h. Isolated yields based on 2a. | |
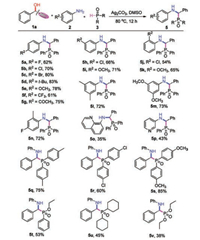
Next, the scope of various amines and H-phosphine oxides was explored. As shown in Scheme 3, both electron-donating and electron-withdrawing aryl amines were suitable for this reaction to give the corresponding products in moderate to good yields (5a-5i). The steric hindrance of substituents on the aryl amines had a slightly effect on the yields of desired products 5j and 5k. Gratifyingly, the di-substituted aryl amines like 3, 5-dimethylaniline, 3, 5-dimethoxyaniline and 4-fluoro-2-methylaniline could afford the corresponding products 5l-5n in good yields. Heterocyclic amines were suitable for this reaction, but leading to the corresponding products 5o and 5p in relatively lower yields. Finally, the compatibility of various H-phosphine oxides was tested. In addition to diphenylphosphine oxide 3a, a series of aryl or alkyl substituted H-phosphine oxides were all compatible for the reaction to produce the corresponding 5q-5u in moderate to good yields. H-Phosphonate such as diethyl phosphite could also be used in this reaction, affording the desired product 5v in relatively lower yield.
|
Download:
|
| Scheme 3. Substrate scope of amines and H-phosphine oxides. Reaction conditions: 1a (0.6 mmol), 2 (0.5 mmol), 3 (1.0 mmol), Ag2CO3 (1.0 mmol), DMSO (4 mL), 80 ℃, 12 h. Isolated yields based on 2. | |
In order to gain insights into the reaction mechanism, several control experiments were carried out (Scheme 4). When radical scavenger 2, 2, 6, 6-tetramethyl-1-piperidinyloxy (TEMPO) was added in the model reaction system, the desired product 4a was still obtained in 72% yield, indicating that a radical process might not be involved in this transformation (Scheme 4a). Furthermore, benzaldehyde 6a was isolated in 17% yield when 1-phenylprop-2-yn-1-ol 1a was separately treated with Ag2CO3 under standard conditon for 3 h (Scheme 4b). This result showed that aldehyde would be formed in the C—C bond cleavage of propargyl alcohol. Moreover, the reaction of imine 7a with diphenylphosphine oxide 3a afforded the corresponding product 4a in 96% yield, suggesting that imine intermediate might be involved in the present reaction system (Scheme 4c). When the reaction of 1-phenylprop-2-yn-1-one, aniline (2a) and diphenylphosphine oxide (3a) was carried out under standard conditions, the side-product (Z)-1-phenyl-3- (phenylamino)prop-2-en-1-one 8a was isolated in 46% yield and product 4a was not observed (Scheme 4d). Furthermore, the reaction did not occur when 1, 3-diphenylprop-2-yn-1-ol or 1-phenylbut-2-yn-1-ol was used in the present reaction system (Scheme 4e). The above results indicated that terminal alkyne and hydroxy group of propargyl alcohols should playa key role in silver mediated C—C bond cleavage of propargylic alcohols.
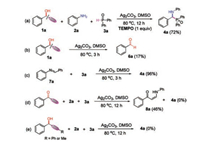
|
Download:
|
| Scheme 4. Control experiments. | |
Based on the experimental results and previous reports [14], a possible reaction pathway was proposed for the synthesis of α-aminophosphine oxides as shown in Scheme 5. Firstly, the reaction of 1-phenylprop-2-yn-1-ol 1a with Ag2CO3 might lead to the formation of alkynyl silver, which further underwent C—C bond cleavage to generate benzaldehyde 6a under heating conditions. Next, the condensation reaction of benzaldehyde 6a with aniline 2a generated imine intermediate 7a. Finally, the addition of diphenylphosphine oxide 3a to imine 7a afforded the desired product 4a.
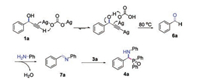
|
Download:
|
| Scheme 5. Possible reaction pathway. | |
In summary, we have developed a new and efficient silvermediated three-component reaction of propargyl alcohols, aromatic amines, and H-phosphine oxides leading to α-aminophosphine oxides. The present aminophosphinoylation reaction could be achieved in one pot operation via sequential C—C and C—O bond cleavage of propargyl alcohols. This strategy provides an attractive and alternative approach to construct α-aminophosphine oxides from simple and readily available materials with wide substrate scope and good functional group tolerance. Further synthetic application and mechanistic investigation is ongoing in our group.
AcknowledgmentsThis work was supported by the Scientific Research Foundation of Zunyi Medical University, the Joint Funds of the Southwest Medical University & Luzhou Municipal Government of China (No. 2018LZXNYD-ZK39), and the Natural Science Foundation of Shandong Province (No. ZR2018MB009).
Appendix A. Supplementary dataSupplementary material related to this article can be found, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2019.07.049.
| [1] |
(a) T. Wang, N. Jiao, Acc. Chem. Res. 47 (2014) 1137-1145; (b) X. Yang, G. Cheng, J. Shen, et al., Org. Chem. Front. 2 (2015) 366-368; (c) L.Y. Xie, S. Peng, L.L. Jiang, et al., Org. Chem. Front. 2 (2019) 167-171; (d) X.H. Ouyang, R.J. Song, J.H. Li, Chem. -Asian J. 13 (2018) 2316-2332; (e) T. Yuan, C. Pi, C. You, et al., Chem. Commun. 55 (2019) 163-166; (f) J.S. Li, Q. Yang, G.Q. Chen, et al., ChemistrySelect 3 (2018) 10621-10623; (g) Y. Yu, Y. Feng, R. Chauvin, et al., Org. Lett. 20 (2018) 4209-4212. |
| [2] |
(a) R. Ren, H. Zhao, L. Huan, et al., Angew. Chem. Int. Ed. 54 (2015) 12692-12696; (b) H. Zhao, X. Fan, J. Yu, et al., J. Am. Chem. Soc. 137 (2015) 3490-3493; (c) J.Y. Wang, P. Zhou, G. Li, et al., Org. Lett. 19 (2017) 6682-6685; (d) X. Wu, C. Zhu, Chem. Rec. 18 (2018) 587-598. |
| [3] |
(a) R.E. Whittaker, G. Dong, Org. Lett. 17 (2015) 5504-5507; (b) K. Chen, S. Liu, D. Wang, et al., J. Org. Chem. 82 (2017) 11524-11530; (c) J. Shen, X. Wang, X. Lin, et al., Org. Lett. 18 (2016) 1378-1381; (d) C. Wu, H.J. Xiao, S.W. Wang, et al., ACS Sustain. Chem. Eng. 7 (2019) 2169-2175; (e) C. Wu, L. Hong, H. Shu, et al., ACS Sustain. Chem. Eng. 7 (2019) 8798-8803; (f) W.H. Bao, M. He, J.T. Wang, et al., J. Org. Chem. 84 (2019) 6065-6071; (g) K.J. Liu, S. Jiang, L.H. Lu, et al., Green Chem. 20 (2018) 3038-3043. |
| [4] |
(a) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30 (2019) 2259-2262; (b) J.S. Li, D.M. Fu, Y. Xue, et al., Tetrahedron 71 (2015) 2748-2752; (c) J.S. Li, F.F. Cai, Z.W. Li, et al., RSC Adv. 4 (2014) 54039-54042; (d) X.M. Xu, D.M. Chen, Z.L. Wang, Chin. Chem. Lett. 31 (2020) 49-57; (e) J.S. Li, Q. Yang, F. Yang, et al., Org. Biomol. Chem. 16 (2016) 140-145; (f) J.S. Li, Y.D. Da, G.Q. Chen, et al., ChemistrySelect 2 (2017) 1770-1773. |
| [5] |
(a) S. Muthusamy, A. Balasubramani, E. Suresh, Org. Biomol. Chem. 16 (2018) 756-764; (b) Y. Li, X. Luo, Y. Shao, L. Chen, J. Org. Chem. 83 (2018) 8768-8774. |
| [6] |
(a) Y. Nishibayashi, Synthesis (2012) 489-503; (b) J. Moran, M. Dryzhakov, E. Richmond, Synthesis 48 (2016) 935-959; (c) R. Roy, S. Saha, RSC Adv. 8 (2018) 31129-31193. |
| [7] |
(a) Y. Zhu, L. Sun, P. Lu, Y. Wang, ACS Catal. 4 (2014) 1911-1925; (b) D. Roy, P. Tharra, B. Baire, Asian J. Org. Chem. 7 (2018) 1015-1032; (c) Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. 30 (2019) 1361-1368. |
| [8] |
(a) Y.W. Kang, Y.J. Cho, K.Y. Ko, et al., Catal. Sci. Technol. 5 (2015) 3931-3934; (b) T. Li, Z. Wang, M. Zhang, et al., Chem. Commun. 51 (2015) 6777-6780; (c) Z. Liu, Y. Xia, S. Feng, et al., Org. Chem. Front. 3 (2016) 1691-1698. |
| [9] |
(a) H. Hu, F. Yang, Y. Wu, J. Org. Chem. 78 (2013) 10506-10511; (b) X. Li, S. Sun, F. Yang, et al., Org. Biomol. Chem. 13 (2015) 2432-2436; (c) F. Sun, M. Li, Z. Gu, Org. Chem. Front. 3 (2016) 309-313; (d) F. Chang, Y. Liu, Syn. Commun. 47 (2017) 961-967; (e) Z. Mi, J. Tang, Z. Guan, et al., Eur. J. Org. Chem. 2018 (2018) 4479-4482. |
| [10] |
(a) L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 1237-1240; (b) C. Wu, J. Wang, X.Y. Zhang, et al., Org. Biomol. Chem. 16 (2018) 5050-5054; (c) P. Bao, L. Wang, Q. Liu, et al., Tetrahedron Lett. 60 (2019) 214-218; (d) C. Wu, L.H. Lu, A.Z. Peng, et al., Green Chem. 20 (2018) 3683-3688; (e) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31 (2020) 67-70; (f) J. Chen, C.H. Ouyang, T. Xiao, H. Jiang, J.S. Li, ChemistrySelect 4 (2019) 7327-7330; (g) F.L. Zeng, X.L. Chen, S.Q. He, et al., Org. Chem. Front. 6 (2019) 1476-1480; (h) L. Wang, Y. Zhang, M. Zhang, et al., Tetrahedron Lett. 60 (2019) 1845-1848; (i) T.Y. Shang, L.H. Lu, Z. Cao, et al., Chem. Commun. 55 (2019) 5408-5419. |
| [11] |
(a) Q. Fu, D. Yi, Z. Zhang, et al., Org. Chem. Front. 4 (2017) 1385-1389; (b) M.S. Li, Q. Zhang, D.Y. Hu, et al., Tetrahedron Lett. 57 (2016) 2642-2646; (c) D. Yi, Q. Fu, S.Y. Chen, et al., Tetrahedron Lett. 58 (2017) 2058-2061; (d) Z.J. Zhang, D. Yi, Q. Fu, et al., Tetrahedron Lett. 58 (2017) 2417-2420; (e) W. Liang, Z. Zhang, D. Yi, et al., Chin. J. Chem. 35 (2017) 1378-1382; (f) D.Y. Hu, M.S. Li, W.W. Zhong, et al., Chin. Chem. Lett. 27 (2016) 1691-1695; (g) C. Liu, W. Wei, D. Yang, et al., Tetrahedron 71 (2015) 6901-6906; (h) C. Liu, M. Zhu, W. Wei, et al., Org. Chem. Front. 2 (2015) 1356-1360; (i) W.W. Zhong, Q. Zhang, M.S. Li, et al., Synth. Commun. 46 (2016) 1377-1385; (j) Q. Huang, K. Dong, W. Bai, et al., Org. Lett. 21 (2019) 3332-3336. |
| [12] |
(a) W. Wei, P. Bao, H. Yue, et al., Org. Lett. 20 (2018) 5291-5295; (b) H. Cui, W. Wei, D. Yang, et al., Green Chem. 19 (2017) 3520-3524; (c) W. Wei, X. Liu, D. Yang, et al., Tetrahedron Lett. 56 (2015) 1808-1811; (d) W. Wei, J. Wen, D. Yang, et al., RSC Adv. 5 (2015) 4416-4419; (e) W. Wei, J. Li, D. Yang, et al., Org. Biomol. Chem. 12 (2014) 1861-1864; (f) H. Cui, W. Wei, D. Yang, et al., Green Chem. 19 (2017) 3520-3524; (g) W. Wei, H. Cui, H. Yue, et al., Green Chem. 20 (2018) 3197-3202; (h) Q. Liu, L. Wang, H. Yue, et al., Green Chem. 21 (2019) 1609-1603. |
| [13] |
(a) Y.C. Guo, J. Li, J.L. Ma, et al., Chin. Chem. Lett. 26 (2015) 755-758; (b) X.F. Zhu, J. Zhang, S. Sun, et al., Chin. Chem. Lett. 28 (2017) 1514-1518; (c) M.K. Awad, M.F. Abdel-Aal, F.M. Atlam, et al., J. Mol. Struct.1173 (2018) 128-141; (d) C. Zhu, D. Wei, Y. Wu, et al., J. Alloys. Compd. 778 (2019) 731-740; (e) Y. You, K. Zhou, B. Guo, et al., ACS Sens. 4 (2019) 774-779; (f) Y. Sheng, Y. You, Z. Cao, et al., Analyst 143 (2018) 2411-2415. |
| [14] |
(a) G. Keglevich, E. Balint, Molecules 17 (2012) 12821-12835; (b) X.A. Li, J.Y. Li, B. Yang, et al., RSC Adv. 4 (2014) 39920-39923; (c) S.A.R. Mulla, M.Y. Pathan, S.S. Chavan, et al., RSC Adv. 4 (2014) 7666-7672. |
 2020, Vol. 31
2020, Vol. 31