b CAS Key Laboratory of Standardization and Measurement for Nanotechnology, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology (NCNST), Beijing 100190, China;
c University of Chinese Academy of Sciences, Beijing 100190, China;
d Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100149, China
The exploration of molecular self-assemblies at surface is an effective way to study and construct the photoelectronic related devices. The star-shaped oligofluorenes (StOFs) with π-conjugated structures have been widely studied, on account of their physicochemical properties and the applications in a wide range of fields [1-3]. For example, as one type of organic light-emitting diodes (OLED) materials, the blue light-emitting StOFs molecules can be utilized in the field of light-emitting devices [4]. Besides, the controllability and enhancement of the performances significantly rely on the self-assemblies of the chemical molecules [5, 6]. Thus, it is of great value to study and regulate such molecular selfassemblies.
In most molecular self-assembled systems, the molecular nanostructures are developed with the help of noncovalent molecular interactions, such as hydrogen bonds, van der Waals interactions, π-π interactions, electrostatic interactions, metalligand coordination interactions and so forth [7-13]. Among them, hydrogen bonds are the most commonly adopted strategy to improve the intermolecular interactions and the stability of the molecular self-assemblies [8, 14]. Besides, carboxyl groups and hydroxyl groups are the constantly designed functional groups that introduced to self-assembled systems to form hydrogen bonds between the molecules. In addition to hydrogen bonds, alkyl chains also play important roles in the molecular self-assembly process. For example, they could provide flexibility to the self-assembled molecular networks [15]. At the same time, the monolayer of the molecular self-assemblies would be more stable via the van der Waals interactions between the alkyl chains and the interactions between the adsorbates and the substrate [8], especially for those with poor coplanarity [4, 14]. Based on the factors mentioned above, the self-assembled structures of StOF-COOH3 [4] with terminal carboxyl groups substituted have been studied on the highly oriented pyrolytic graphite (HOPG) surface with the aid of scanning tunneling microscopy (STM) [16]. STM characterization is an efficient method with high spatial resolution for direct identification and deep investigation of individual chemical structures in sub-molecular or atomic scale [16]. The StOF-COOH3 molecules finally developed into two-dimensional (2D) porous honeycomb networks and reached the dynamic equilibrium on the HOPG surface through hydrogen bonds and van der Waals interactions. In the past few years, there existed a great deal of studies related to the StOF-COOH3 molecular self-assemblies and structural modulations [7, 13, 14, 17-21]. But till now the innovation of the 2D ordered molecular architectures is still a challenge for the controlled molecular self-assemblies and nano-scale structural engineering.
In order to explore novel self-assembly strategies, scientists have investigated the covalent coupled structures as well [22-25], except for the noncovalent molecular self-assemblies. Among those, D. Peyrot et al. have discovered the competitive adsorption of the halogen bonded self-assemblies and the covalent coupled structures of molecule 1, 3, 5-tris(4-iodophenyl)benzene [26]. The covalent conjugations appearing at the step edge only covered a small area of the surface. Compared with that, the 2D halogen bonded self-assemblies preferred to grow more on the surface. On the other hand, the 2D halogen bonded self-assembled architectures with less defects were more compact, which helped decreasing the surface free energy and improving the stability of the molecular system. Thus, the interaction between the halogen atoms has emerged as one potential candidate for the noncovalent construction of 2D supramolecular self-assembled networks and crystal engineering [26-30]. Z. Guo et al. have reported the different self-assembled nanoarchitectures of molecule N-(1-dodecyl)-perylene-3, 4-dicarboxmonoimide (PMI) and the bromine atoms substituted molecule N-(1-dodecyl)-9-bromoperylene-3, 4-dicarboxmonoimide (Br-PMI) [28], which verified the existence of halogen…halogen interactions. Besides, the interaction between the halogen atoms could also contribute to the stability of the self-assembled molecular systems.
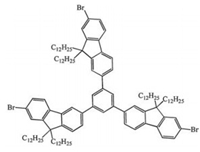
In this work, we designed and synthesized the star-shaped molecule StOF-Br3 (Fig. 1, the experimental details can be seen in the Supporting Information), which is an analogue of the classic molecule StOF-COOH3 with all the carboxyl groups substituted by bromine atoms. On the basis of STM characterization, we obtained the high-resolution images of the molecular self-assemblies, in which we observed the detailed structural information of the ordered molecular nanostructures with large scale. According to the density functional theory (DFT) calculation and the comparison of molecular nanostructures between StOF-Br3 and StOF-COOH3, we figured out the self-assembled mechanisms of StOF-Br3 molecular nanoarchitectures. The halogen…halogen interaction could contribute to the stability of the self-assembled molecular system, which plays an important role for developing the ordered molecular arrangements with large scale. Meanwhile, the interaction between the halogen atoms could be proposed as a new bottom-up strategy for constructing different structural nanoarchitectures with large areas.
|
Download:
|
| Fig. 1. Chemical structure of StOF-Br3, R = C12H25. | |
In order to verify the self-assembled architectures of molecule StOF-Br3, a series of STM characterizations were performed. As the concentration of the molecular solution could affect the selfassembled nanostructures of the molecules [21, 31-33], the STM characterizations of StOF-Br3 molecular self-assemblies were performed using the higher concentration (1 × 10-4 mol/L) and the lower concentration (3 × 10-5 mol/L). The STM images of different StOF-Br3 self-assembled structures are shown in Fig. 2. Fig. 2a corresponds to the StOF-Br3 self-assemblies in lower concentration, and the bright dots in the picture with higher local electron cloud density are determined to be the backbones of molecule StOF-Br3, which are labelled by red arrows. The distance between two bright dots was measured to be about 2.2 ± 0.1 nm, which is a little far for the formation of any hydrogen bond. When we performed the characterizations of the StOF-Br3 molecular self-assemblies with higher concentration, we obtained the selfassembled STM image as shown in Fig. 2b. From the picture we can distinguish that the backbones of molecule StOF-Br3 get closer to each other in one row and present like a wave, as indicated by the red line in Fig. 2b. Compared with the backbones of the molecule, the alkyl chains in StOF-Br3 show lower brightness and could not be distinguished from the STM image, which is due to the lower local electron density. We use cartoon models to exhibit the arrangements of the StOF-Br3 molecular self-assemblies, as shown in Fig. 2c. The dark blue star-shaped branches represent the backbones of the star-shaped molecule StOF-Br3, and the orange balls represent the terminal bromine atoms. The cartoon models can explain why the StOF-Br3 molecules arrange like waves that we observed from the STM image. The backbones are very similar to that of the molecule 1, 3, 5, -tris(4-iodophenyl)benzene [26], but we obtained the different molecular arrangements. The tunneling conditions corresponding to different molecular self-assembled STM images are presented in the figure legends.

|
Download:
|
| Fig. 2. STM images of the self-assembled molecule StOF-Br3 at heptanoic acid/HOPG surface. (a) Lower concentration, scan size: 48.47 nm × 48.47 nm, tunneling conditions: Iset = 289.9 pA, Vbias =594.5 mV; (b) Higher concentrations, scan size: 45.47 nm × 45.47 nm, tunneling conditions: Iset = 165.4 pA, Vbias =1161 mV; (c) Cartoon model of the assembled architecture. The skeletons of molecule StOF-Br3 are presented by dark blue star-shaped branches, and the bromine atoms are labelled by orange balls. The red line shows the arrangement of the molecule configuration in one row. | |
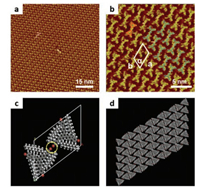
Compared with that in lower concentration, the StOF-Br3 selfassembled nanostructures with higher concentration showed more advantages for the structural constructions of functional materials, since they were more compact and ordered. Thus, we further investigated the detailed information of the self-assemblies of StOF-Br3 molecular nanostructures with higher concentration and different tunneling conditions, as it could provide different electron informations of the self-assembled STM images with different tunneling conditions. The STM images in Fig. 3 correspond to the StOF-Br3 molecular self-assemblies at low voltages. As shown in Fig. 3a, the StOF-Br3 molecules at the heptanoic acid/HOPG surface were very stable and developed into lamellar structures. Besides, the highly ordered and closely packed non-porous arrangements with large area can be observed. The high-resolution STM image of the molecular self-assemblies is presented in Fig. 3b, from which we can observe that the molecules are arranged in pairs. Each pair of molecules is composed of two trigons, which are the backbones of two opposite StOF-Br3 molecules labelled orange in the image. One StOF-Br3 molecule can cover the other one in the molecular pairs after rotating 180°. When observing the molecules carefully, we can distinguish that each molecule is consist of three bright dots, which represent three fluorene arms with terminal bromine atoms in the molecule StOFBr3, as the electron density is comparatively high. Correspondingly, the central phenyls and the alkyl chains exhibit relatively dark characteristics. Each bright dot was measured to be 0.7 ± 0.1 nm, which is a little longer compared with that of molecule StOFCOOH3 [4], as the bromine atom in the STM image is bright and counted as one part of the bright dot. The unit cell of the molecular ordered arrangements is labelled in Fig. 3b. The parameter a is measured to be 4.6 ± 0.1 nm, b is measured to be 2.8 ± 0.1 nm, and the angle α between the two directions is determined to be 57 ± 1°. Besides, the parameters calculated by DFT are as follows: a = 4.52 nm, b = 2.96 nm, and α = 55°. From the comparison we can conclude that the experimental data are consist with that of the calculation results. The molecular models of the stripe pairs are labelled blue, and can fully overlap the scanned molecular selfassemblies, as indicated in Fig. 3b. The alkyl chains are omitted, as the local electron density is rather lower than other portions of the molecule. Besides, there is no solvent molecule arranged between the dark gullies of the assembled StOF-Br3 molecules. As proved by the DFT calculations, the total energy per unit area of the molecular StOF-Br3 self-assemblies is rather low (Table 1), which shows the stability of the StOF-Br3 self-assembled molecular system without any co-assembled heptanoic acid molecule.
|
Download:
|
| Fig. 3. STM images and the molecular models of self-assemblies of StOF-Br3 at the heptanoic acid/HOPG surface. (a) Large scale STM image of the StOF-Br3 selfassemblies, scan size: 78.35 nm × 78.35 nm, tunneling conditions: Iset = 299.1 pA, Vbias =699.8 mV; (b) High-resolution STM image of the StOF-Br3 self-assemblies, scan size: 22.29 nm × 22.29 nm, tunneling conditions: Iset = 299.1 pA, Vbias =699.8 mV; (c) Molecular model of the StOF-Br3 dimer, the bromine-bromine interaction is indicated by the yellow circle; (d) Molecular models of StOF-Br3 selfassemblies. | |
|
|
Table 1 The corresponding energies of the adsorbates on HOPG surface calculated by DFT method. |
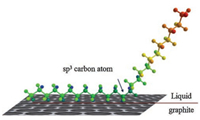
The distance between the two opposite bromine atoms in one pair was measured to be about 0.2 nm, which indicates the existence of the intermolecular bromine-bromine interactions between the two opposite bromine atoms in one pair, as shown by the yellow circle in Fig. 3c. The structural models of the selfassemblies and the arrangements of the molecules are exhibited in Fig. 3d. In order to improve the molecule-molecule and moleculesubstrate interactions, alkyl chains were introduced to the molecular system. As the alkyl chains were unfixed, not all the chains could self-assemble at the surface. As shown in Fig. 4, only half of the alkyl chains are adsorbed on the substrate, while the rest of the chains are extending in the solution.
|
Download:
|
| Fig. 4. Configuration of the alkyl chains at the sp3 carbon atom of the StOF part on the liquid/graphite interface. | |
In order to further explain the forming mechanisms of the StOF-Br3 self-assembled molecular architectures, we carried on the DFT calculations. The calculated energies corresponding to the process of the self-assemblies are presented in Table 1. The energy between the StOF-Br3 molecules and the substrate is about -152.304 kcal/mol, which is comparable with that of StOF-COOH3 molecular system, as the stability between the molecules and the substrate is mainly contributed by the intermolecular interactions between the aromatic rings and alkyl chains. In the StOF-Br3 molecular self-assemblies, only one bromine atom interacted with the opposite one in the dimer, so the energy between the StOF-Br3 molecules could be a little higher, which is calculated to be -22.990 kcal/mol. The total energy of StOF-Br3 is accordingly larger, as is reported that halogen…halogen interactions may be a little weaker than that of the hydrogen bonds. The total energy per unit area of StOF-Br3 is calculated to be -0.160 kcal/mol, which is comparable and even lower than that of the StOF-COOH3 molecular system. The reason may be that the closely packed architecture of StOF-Br3 is much more compact than that of StOFCOOH3. In general, molecule StOF-Br3 self-assembled into the closely packed and ordered arrangements in a two-step process. Firstly, the molecules self-assembled into dimers through the Br…Br interactions. And then the dimers arranged themselves into rows through the van der Waals interactions between the alkyl chains. Combined with the interactions between the StOFBr3 molecules and the substrate, the StOF-Br3 molecular architectures reached the dynamic balance.
The DFT calculations verified that the Br…Br intermolecular interaction turned out to be strong enough and mainly controlled the process of the StOF-Br3 molecular self-assemblies. With the comprehensive interactions between the molecules and the substrate, the StOF-Br3 molecules developed into a highly ordered and closely packed molecular nanoarchitecture. Similar with hydrogen bonds, the halogen…halogen interactions can be formed simultaneously, which could introduce a new binding strategy for the quick bottom-up structural constructions with different geometries.
In summary, we came up a new kind of star-shaped chemical molecule StOF-Br3 with three bromine atoms at the end of fluorene arms. The self-assemblies of the halogen bonded molecular arrangements were characterized by STM at the heptanoic acid/HOPG surface. From the high-resolution self-assembled STM image, we observed the highly ordered and closely packed arrangements, and distinguished the detailed structural information of the individual StOF-Br3 molecule. Combined with DFT calculations and the surface free energies, we figured out the self-assembly mechanisms of StOF-Br3 molecular nanoarchitectures. Meanwhile, the halogen…halogen interaction turned out to be strong enough to stabilize the ordered molecular self-assemblies, which provides a kind of effective approach for the quick building of molecular nanoarchitectures with large areas and different geometries.
AcknowledgmentsThe financial supports from the National Natural Science Foundation of China (NSFC, Nos. 21773041 and 21327805) and the National Basic Research Program of China (No. 2016YFA0200700) are gratefully acknowledged.
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.07.029.
| [1] |
A. Miura, P. Jonkheijm, S. De Feyter, et al., Small 1 (2005) 131-137. |
| [2] |
Y. Shirota, J. Mater. Chem. 10 (2000) 1-25. DOI:10.1039/a908130e |
| [3] |
K. Okumoto, Y. Shirota, Chem. Mater. 15 (2003) 699-707. DOI:10.1021/cm020849+ |
| [4] |
Z. Ma, Y.Y. Wang, P. Wang, et al., ACS Nano 1 (2007) 160-167. DOI:10.1021/nn7000678 |
| [5] |
H. Sirringhaus, P.J. Brown, R.H. Friend, et al., Nature 401 (1999) 685-688. DOI:10.1038/44359 |
| [6] |
L.A. Bumm, J.J. Arnold, M.T. Cygan, et al., Science 271 (1996) 1705-1707. DOI:10.1126/science.271.5256.1705 |
| [7] |
Y. Li, Z. Ma, K. Deng, et al., Chem. -Eur. J. 15 (2009) 5418-5423. DOI:10.1002/chem.200900493 |
| [8] |
S. De Feyter, F.C. De Schryver, Chem. Soc. Rev. 32 (2003) 139-150. DOI:10.1039/b206566p |
| [9] |
K.S. Mali, K. Lava, K. Binnemans, et al., Chem. -Eur. J. 16 (2010) 14447-14458. DOI:10.1002/chem.201001653 |
| [10] |
R. Gutzler, S. Lappe, K. Mahata, et al., Chem. Commun. (2009) 680-682. |
| [11] |
J.M. MacLeod, O. Ivasenko, C. Fu, et al., J. Am. Chem. Soc. 131 (2009) 16844-16850. DOI:10.1021/ja906206g |
| [12] |
M. Marschall, J. Reichert, A. Weber-Bargioni, et al., Nat. Chem. 2 (2010) 131-137. DOI:10.1038/nchem.503 |
| [13] |
X. Zhang, Q. Zeng, C. Wang, RSC Adv. 3 (2013) 11351-11366. DOI:10.1039/c3ra40473k |
| [14] |
Y. Li, Z. Ma, G. Qi, et al., J. Phys. Chem. C 112 (2008) 8649-8653. |
| [15] |
J. Lu, S.B. Lei, Q.D. Zeng, et al., J. Phys. Chem. B 108 (2004) 5161-5165. |
| [16] |
G. Binning, H. Rohrer, C. Gerber, et al., Phys. Rev. Lett. 49 (1982) 57-61. DOI:10.1103/PhysRevLett.49.57 |
| [17] |
J. Xu, Q.D. Zeng, Curr. Org. Chem. 18 (2014) 407-415. DOI:10.2174/13852728113176660153 |
| [18] |
X. Zhang, Q. Zeng, C. Wang, Sci. China Chem. 57 (2014) 13-25. DOI:10.1007/s11426-013-4975-9 |
| [19] |
M. Li, Q. Zeng, C. Wang, Sci. China Phys. Mech. 54 (2011) 1739-1748. DOI:10.1007/s11433-011-4482-y |
| [20] |
S. Wu, F. Zhao, S. Luo, et al., J. Nanosci. Nanotechnol. 17 (2017) 725-728. DOI:10.1166/jnn.2017.12402 |
| [21] |
H. Dai, W. Yi, K. Deng, et al., ACS Appl. Mater. Interfaces 8 (2016) 21095-21100. DOI:10.1021/acsami.6b06638 |
| [22] |
S. Weigelt, C. Bombis, C. Busse, et al., ACS Nano 2 (2008) 651-660. DOI:10.1021/nn7004365 |
| [23] |
J.A. Lipton-Duffin, O. Ivasenko, D.F. Perepichka, et al., Small 5 (2009) 592-597. DOI:10.1002/smll.200801943 |
| [24] |
M.I.t. Veld, P. Iavicoli, S. Haq, et al., Chem. Commun. (2008) 1536-1538. |
| [25] |
M. Treier, N.V. Richardson, R. Fasel, J. Am. Chem. Soc. 130 (2008) 14054-14055. DOI:10.1021/ja805342n |
| [26] |
D. Peyrot, F. Silly, ACS Nano 10 (2016) 5490-5498. DOI:10.1021/acsnano.6b01938 |
| [27] |
J.C. Russell, M.O. Blunt, J.M. Garfitt, et al., J. Am. Chem. Soc. 133 (2011) 4220-4223. DOI:10.1021/ja110837s |
| [28] |
Z. Guo, P. Yu, K. Sun, et al., Phys. Chem. Chem. Phys. 19 (2017) 31540-31544. DOI:10.1039/C7CP06027K |
| [29] |
R. Gutzler, O. Ivasenko, C. Fu, et al., Chem. Commun. 47 (2011) 9453-9455. DOI:10.1039/c1cc13114a |
| [30] |
B. Zha, M. Dong, X. Miao, et al., J. Phys. Chem. Lett. 7 (2016) 3164-3170. DOI:10.1021/acs.jpclett.6b01508 |
| [31] |
L. Xu, X.R. Miao, L.H. Cui, et al., J. Phys. Chem. C 119 (2015) 17920-17929. DOI:10.1021/acs.jpcc.5b04799 |
| [32] |
X. Miao, L. Xu, L. Cui, et al., Phys. Chem. Chem. Phys. 16 (2014) 12544-12553. DOI:10.1039/C4CP00871E |
| [33] |
H. Nguyen Thi Ngoc, T.G. Gopakumar, M. Hietschold, Surf. Sci. 607 (2013) 68-73. DOI:10.1016/j.susc.2012.08.008 |
 2020, Vol. 31
2020, Vol. 31 


