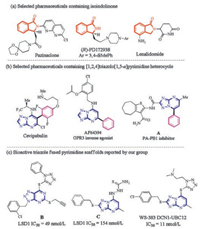
N-Heterocyclic scaffolds, widely found in pharmaceuticals, agrochemicals and natural products, are considered as one of the most abundant and integral motifs due to their interesting biological activities [1-4]. Among these N-heterocyclic compounds, the isoindolinone frameworks are a privileged class of building blocks found in many pharmaceutically relevant molecules, such as Pazinaclone (acting as a partial agonist at GABAA benzodiazepine receptors for the treatment of anxiety) [5], (R)-PD172938 (an antagonist of the dopamine D4 receptor for the treatment of psychosis) [6], and Lenalidomide (an immunomodulatory agent for the treatment of multiple myeloma) [7] (Fig. 1a). In addition, the[1,2,4]triazolo[1, 5-a]pyrimidine fragment is another biologically important scaffold, compounds bearing this scaffold have been found to possess diverse bioactivities [8-10]. Of particular interest are the[1,2,4]triazolo[1, 5-a]pyrimidine containing biaryl compounds, which possess unique structural features ( e.g., chiral center) and interesting biological profiles [11-13]. For instance, Cevipabulin (binding to the vinca domain of tubulin for the treatment of tumors) [14], AF64394 (a selective GPR3 inverse agonist for the treatment of neuropsychiatric disorders) [15], compound A (a PA-PB1 interaction inhibitor for the treatment of influenza) [16] (Fig. 1b). Our group has also identified some biologically important triazole fused pyrimidine derivatives with anticancer potential (Fig. 1c) [17-22]. For example, compound B effectively and reversibly inhibits histone lysine specific demethylase 1 (LSD1/KDM1A) with an IC50 value of 49 nmol/L, and exhibits high selectivity to LSD1 over monoamine oxidases (MAO-A/B) [18]. Compound C inactivates LSD1 (IC50 = 154 nmol/L) and also significantly inhibits migration of lung cancer cells A549 and PC-9 [21]. Very recently, we have found that WS-383 effectively inhibits neddylation of Cul3 and Cul1 in cells by interrupting the DCN1-UBC12 protein-protein interaction (IC50 = 11 nmol/L) [22].
|
Download:
|
| Fig. 1. Bioactive molecules having isoindolinone and triazole-fused pyrimidine cores. | |
In view of the biological importance of the isoindolinone and [1,2,4]triazolo[1, 5-a]pyrimidine containing biaryl substructures, it would be interesting to generate structurally new molecular entities (NMEs) possessing these two substructures through the molecular hybridization approach, which is widely employed in drug discovery [23, 24]. It is generally believed that these hybridized molecules have improved and/or new biological profiles. However, the molecular hybridization approach always relies on pluripotent functional groups ( e.g., -COOH, -NH2, -OH) to form hybridized molecules. Therefore, the question on how to efficiently combine these two frameworks without these functional groups into one molecule has not been fully addressed.
The C(sp3)-H functionalization is an emerging strategy in organic synthesis and has found broad applications in late-state diversification of complex natural products and advanced drug molecules [25, 26]. The methyl group attached to the electrondeficient N-heterocycles have been found to undergo diverse transformations depending on the reaction conditions [27-32]. Following this work, we herein report a Brønsted acid-promoted three-component cascade reaction for the synthesis of structurally complex isoindolinone/[1,2,4]triazolo[1, 5-a]pyrimidine hybrids on water based on the C(sp3)-H functionalization of the methyl group (Scheme 1).

|
Download:
|
| Scheme 1. Our present work on C(sp3)-H functionalization. | |
The trimethoxyphenyl (TMP) group is prevalent in natural products and drug candidates, particularly widely found in tubulin inhibitors [33]. Till date, a large number of TMP-based tubulin inhibitors have been identified, and some of them such as ZD6126, BNC-105p, CKD-516, CA-1 P, CA-4 P, and AVE8062 are currently being evaluated in clinical trials for cancer therapy. Given the prevalence and biological importance of the TMP moiety, 5-methyl-7-(2, 4, 6-trimethoxyphenyl)-[1,2,4]triazolo[1, 5-a]pyrimidine (1a) was utilized with high priority as a model substrate coupled with 2-formylbenzoic acid (2a) and aniline (3a), expecting to obtain biologically interesting TMP containing isoindolinone/[1,2,4]triazolo[1, 5-a]pyrimidine hybrids (Table 1). Firstly, several commonly used solvents were examined (entries 1–7), the results indicated that water was the best solvent for this transformation, forming compound 4a in 43% yield (entry 7). Other organic solvents were less efficient. Based on the possible reaction mechanism, we speculate that addition of acidic additive may promote this conversion. We next examined the effects of different acidic additives on the reactivity (entries 8–14), and found that acetic acid (AcOH) significantly improved the reaction efficiency, compound 4a was formed in 93% yield (entry 14). Other Brønsted acids such as benzoic acid, trifluoroacetic acid (TFA), ptoluenesulfonic acid (TsOH) and trifluoromethanesulfonic acid (TfOH) were less efficient (entries 9–12). Besides, decrease of additive (AcOH) loading led to decreased yield, compound 4a was obtained in 68% yield when using 5 equiv. of AcOH in H2O (entry 13). Considering the high reactivity, the reaction was not further optimized. Based on above optimizations, the best reaction condition was that 1a (1.0 mmol), 2a (1.0 mmol) and aniline (3a, 1.0 mmol) were stirred in H2O (2 mL) at 100 ℃ for 12 h in the presence of AcOH (10 mmol).
|
|
Table 1 Optimization of the reaction conditions.a |
With the optimized reaction conditions in hand, we next examined the scope and generality of this three-component reaction (Scheme 2). The title compounds4a-q were obtained in moderate to excellent yields (67%–96%) regardless of the electronic nature and steric effects of the substituents. It should be noted that halogen atoms such as fluoro, chloro and bromo were well tolerated, and could be potentially used for further functionalization. However, for aliphatic amine substrate, the yield decreased. Compound 4o was generated in 67% yield when glycine ethyl ester was used, suggesting that other amino esters could also be employed to construct structurally diverse and complex compounds for biological screening. Inspired by the reactivity observed, we then examined the reactivity of other electrondeficient N-heterocycles bearing the methyl group. To our delight, compounds 4p and 4q were obtained in 81% and 86% yields, respectively. Of note, heteroaromatic amines were incompatible with this protocol and failed to generate the corresponding products. All new products were fully characterized by 1H and 13C NMR (please see the Supporting information for associated NMR spectra). The current work has provided an example regarding the synthesis of complex molecules based on the C(sp3)-H functionalization. Conceivably, other aliphatic amines and electrondeficient N-heterocycles bearing the activated methyl group could also be used under the optimal condition to generate structurally new compounds.

|
Download:
|
| Scheme 2. Substrate scope. Reaction conditions: 1a (1 mmol), 2a (1 mmol), 3 (1 mmol), AcOH (10 mmol), H2O (2 mL), 12 h, 100 ℃. | |
Based on the above observation, we proposed a plausible mechanistic pathway as depicted in Scheme 3. Initially, the imine intermediate A was formed through the acetic acid promoted condensation of aniline 3a with 2-formylbenzoic acid 2. Followed by protonation, the activated imine B was afforded, the acidity of C—H bond in protonated compound 1a increased, forming the nucleophilic intermediate C, the intermediate C then attacked the activated imine bond, followed by intramolecular cyclization reaction to generate compound 4a. It should be noted that in this process, an isoindolinone framework was constructed, accompanying by formation of consecutive C—C and C—N bonds.

|
Download:
|
| Scheme 3. Proposed mechanism for the formation of 4a. | |
With these compounds in hand, we next tested the inhibitory activity of the synthesized compounds against the SKP2-CKS1 interaction based on our established HTRF method. As shown in Fig. 2, some compounds showed acceptable inhibitory activity. Particularly, compounds 4b, 4j, and 4n exhibited moderate inhibition against the SKP2-CKS1 interaction at 50 μmol/L with the inhibitory rates of 45%, 57%, and 54%, respectively. We believe that these compounds could be used as hit compounds to perform further structure-based design for more potent inhibitors.

|
Download:
|
| Fig. 2. The inhibitory activity of selected compounds against the SKP2-CKS1 interaction. GST-SKP2 and His6-CKS1 were treated with the compounds at 50 μmol/L, and the inhibition rates were determined by the HTRF binding assay. | |
In summary, we have developed an efficient Brønsted acidpromoted C(sp3)-H functionalization approach that enables rapid access to the structurally new isoindolinone/[1,2,4]triazolo[1, 5-a] pyrimidine hybrids on water from 5-methyl-7-(2, 4, 6-trimethoxyphenyl)-[1,2,4]triazolo[1, 5-a]pyrimidine, 2-formylbenzoic acid and various anilines. The title compounds were generated in high to excellent yields (up to 96%) regardless of the electronic nature and steric effects of the substituents. In this reaction, an isoindolinone scaffold, one C—C single bond, and two C—N bonds were formed simultaneously with high atom economy. It is worth noting that the biologically important biaryl, [1,2,4]triazolo[1, 5-a] pyrimidine, and isoindolinone substructures were efficiently incorporated into one molecule through the Brønsted acidpromoted C(sp3)-H functionalization approach. Biological evaluation indicated that some of these compounds showed promising inhibitory activity against the SKP2-CKS1 interaction and therefore could be used to design more potent small-molecule inhibitors targeting the SKP2-CKS1 interaction.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 81773562 and 81703326), China Postdoctoral Science Foundation (Nos. 2018M630840 and 2019T120641), and Scientific Program of Henan Province (No. 182102310123). We sincerely thank Dr. Erqing Li (Zhengzhou University) for analyzing the NMR spectra of some compounds.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.07.019.
| [1] |
E. Vitaku, D.T. Smith, J.T. Njardarson, J. Med. Chem. 57 (2014) 10257-10274. DOI:10.1021/jm501100b |
| [2] |
J. Yu, A. Ciancetta, S. Dudas, et al., J. Med. Chem. 61 (2018) 4860-4882. DOI:10.1021/acs.jmedchem.8b00168 |
| [3] |
M.C. Hilton, R.D. Dolewski, A. McNally, J. Am. Chem. Soc. 138 (2016) 13806-13809. DOI:10.1021/jacs.6b08662 |
| [4] |
L.M. Blair, J. Sperry, J. Nat. Prod. 76 (2013) 794-812. DOI:10.1021/np400124n |
| [5] |
Y. Zhang, H. Zhu, Y. Huang, et al., Org. Lett. 21 (2019) 1273-1277. DOI:10.1021/acs.orglett.8b04026 |
| [6] |
S.K. Ray, M.M. Sadhu, R.G. Biswas, R.A. Unhale, V.K. Singh, Org. Lett. 21 (2019) 417-422. DOI:10.1021/acs.orglett.8b03597 |
| [7] |
X. Qiu, N. Sun, Y. Kong, et al., Org. Lett. 21 (2019) 3838-3841. DOI:10.1021/acs.orglett.9b01326 |
| [8] |
S.D. Kim, H.N. Kim, J.H. Lee, et al., Biochem. Pharmacol. 86 (2013) 782-790. DOI:10.1016/j.bcp.2013.07.015 |
| [9] |
B. Kim, J.H. Lee, W.J. Jin, et al., Cell. Signal. 49 (2018) 68-78. DOI:10.1016/j.cellsig.2018.06.001 |
| [10] |
S. Massari, J. Desantis, G. Nannetti, et al., Org. Biomol. Chem. 15 (2017) 7944-7955. DOI:10.1039/C7OB02085F |
| [11] |
G. Bringmann, T. Gulder, T.A.M. Gulder, M. Breuning, Chem. Rev. 111 (2011) 563-639. DOI:10.1021/cr100155e |
| [12] |
Y. Yang, J. Lan, J. You, Chem. Rev. 117 (2017) 8787-8863. DOI:10.1021/acs.chemrev.6b00567 |
| [13] |
J.A. Garcia-Lopez, M.F. Greaney, Chem. Soc. Rev. 45 (2016) 6766-6798. DOI:10.1039/C6CS00220J |
| [14] |
K. Oukoloff, J. Kovalevich, A.S. Cornec, et al., Bioorg. Med. Chem. Lett. 28 (2018) 2180-2183. DOI:10.1016/j.bmcl.2018.05.010 |
| [15] |
T. Jensen, L. Elster, S.M. Nielsen, et al., Bioorg. Med. Chem. Lett. 24 (2014) 5195-5198. DOI:10.1016/j.bmcl.2014.09.077 |
| [16] |
S. Massari, G. Nannetti, J. Desantis, et al., J. Med. Chem. 58 (2015) 3830-3842. DOI:10.1021/acs.jmedchem.5b00012 |
| [17] |
S. Wang, Z.R. Li, F.Z. Suo, et al., Eur. J. Med. Chem. 167 (2019) 388-401. DOI:10.1016/j.ejmech.2019.02.039 |
| [18] |
Z. Li, L. Ding, Z. Li, et al., Acta Pharm. Sinica B 9 (2019) 794-808. DOI:10.1016/j.apsb.2019.01.001 |
| [19] |
Z.R. Li, S. Wang, L. Yang, et al., Eur. J. Med. Chem. 166 (2019) 432-444. DOI:10.1016/j.ejmech.2019.01.075 |
| [20] |
B. Yu, X.J. Shi, Y.F. Zheng, et al., Eur. J. Med. Chem. 69 (2013) 323-330. DOI:10.1016/j.ejmech.2013.08.029 |
| [21] |
S. Wang, L.J. Zhao, Y.C. Zheng, et al., Eur. J. Med. Chem. 125 (2017) 940-951. DOI:10.1016/j.ejmech.2016.10.021 |
| [22] |
S. Wang, L. Zhao, X.J. Shi, et al., J. Med. Chem. 62 (2019) 2772-2797. DOI:10.1021/acs.jmedchem.9b00113 |
| [23] |
V.J. Claudio, D. Amanda, B. Vanderlan da Silva, J.B. Eliezer, F. Carlos Alberto Manssour, Curr. Med. Chem. 14 (2007) 1829-1852. DOI:10.2174/092986707781058805 |
| [24] |
B. Yu, P.P. Qi, X.J. Shi, et al., Eur. J. Med. Chem. 117 (2016) 241-255. DOI:10.1016/j.ejmech.2016.04.024 |
| [25] |
T. Cernak, K.D. Dykstra, S. Tyagarajan, P. Vachal, S.W. Krska, Chem. Soc. Rev. 45 (2016) 546-576. DOI:10.1039/C5CS00628G |
| [26] |
J. He, L.G. Hamann, H.M.L. Davies, R.E.J. Beckwith, Nat. Commun. 6 (2015) 5943. DOI:10.1038/ncomms6943 |
| [27] |
S. Yuan, B. Yu, H.M. Liu, Adv. Synth. Cat. 361 (2019) 59-66. DOI:10.1002/adsc.201801226 |
| [28] |
S.A.R. Mulla, M.Y. Pathan, S.S. Chavan, RSC Adv. 3 (2013) 20281-20286. DOI:10.1039/c3ra43515f |
| [29] |
X. Gao, F. Zhang, G. Deng, L. Yang, Org. Lett. 16 (2014) 3664-3667. DOI:10.1021/ol501422k |
| [30] |
P. Kohls, D. Jadhav, G. Pandey, O. Reiser, Org. Lett. 14 (2012) 672-675. DOI:10.1021/ol202857t |
| [31] |
S.V.N. Vuppalapati, Y.R. Lee, Tetrahedron 68 (2012) 8286-8292. DOI:10.1016/j.tet.2012.07.051 |
| [32] |
F.F. Wang, C.P. Luo, Y. Wang, G. Deng, L. Yang, Org. Biomol. Chem. 10 (2012) 8605-8608. DOI:10.1039/c2ob26604k |
| [33] |
L. Li, S. Jiang, X. Li, et al., Eur. J. Med. Chem. 151 (2018) 482-494. DOI:10.1016/j.ejmech.2018.04.011 |
 2020, Vol. 31
2020, Vol. 31 


