Thioether spirals are a class of important structural motifs with wide applications in organic synthesis, medicinal chemistry and material chemistry, and their synthesis methods have aroused great interests from relevant workers [1-8]. Recently, organic dyes or transition metal salts have been applied in photocatalytic or thermal reactions to obtain target products. For example, Eosin Y sodium was used as a photocatalyst for the synthesis of 3-sulfonyl and 3-sulfenyl azaspiro[4, 5]trienones [9], followed by developing a AgCl catalyst in spirocyclization reaction to get 3-thioazaspiro[4, 5]trienones, a key product in biology (Scheme 1) [10]. However, these homogeneous catalysts are difficult to be separated from the reaction solutions thus their recovery take additional energy. Moreover, the reaction substrate aryl benzene requires a para-methoxy group or a methyl group and the yields of 3-sulfenyl azaspiro[4, 5]trienones obtained by these methods is low [11-16]. Hence, it is necessary to develop an efficient synthetic method in a greener way for spirocyclization. In recent years, noble metal nanoparticles (NPs) have attracted great attentions as a photocatalyst in driving chemical reactions [17]. It has been found that palladium (Pd) NPs can efficiently convert visible light to chemical energy in fine organic synthesis at ambient temperatures [18]. Considering visible light occupies a large fraction (~43%) of solar energy, the visible light photocatalysis is a green and promising way in driving chemical reactions [19]. Besides, the visible light is help for forming carbon-carbon and carbon heteroatom bonds [20-30] via catalytic oxidation route in molecules assembling. In addition, the visible-light-induced electrons on the metal NPs surface can be directly transferred to the anti-bonding orbital of adsorbed molecules, which enhances the intrinsic catalytic activity of metal NPs and triggers the reaction significantly. When the noble metal NPs are supported on a photocatalytic inert carrier, it can be reused as a visible light photocatalyst with stable performance. Herein, we present the spirocyclization reaction of alkyne can be conducted effectively at mild conditions through using supported Pd NPs as the photocatalyst under visible light (Scheme 1).

|
Download:
|
| Scheme 1. Various routes for the spirocyclization reactions. | |
We characterized the catalyst Pd/ZrO2 by XRD, TEM and UV–vis. Initially, the TEM image (Fig. 1A) demonstrates palladium nanoparticles (Pd NPs) of spherical particles dispersed on ZrO2. The particle size of Pd NPs supported on ZrO2 is less than 8 nm, and most are 4–7 nm. This indicates that the catalyst is a nanoscale catalyst (Fig. 1B). Then, it can be seen from XRD that the introduction of the preparation method of Pd has no effect on the structure of ZrO2. No characteristic diffraction peaks were observed in the XRD spectrum, possibly due to the low contents of Pd or Pd forming amorphous nanoparticles. Finally, the UV–vis spectrum (Fig. 1C) shows that the absorption of Pd/ZrO2 is stronger than that of ZrO2 in the ultraviolet and visible range, indicating that the enhanced light absorption is due to the dispersed ZrO2 surface of the Pd nanoparticles.

|
Download:
|
| Fig. 1. (A) TEM image of 3 wt% Pd/ZrO2. (B) The X-ray diffraction patterns for pure 3 wt% Pd/ZrO2 (red) and ZrO2 (black). (C) UV–vis spectra of pure ZrO2 (black), 3 wt% Pd/ZrO2 (red). | |
In our preliminary investigation, we investigated the different reaction conditions under the induction of visible light with 1a and 2a as the reaction substrates. The results were summarized in Table 1. The solvents such as DMF, ethanol, dioxane and THF were filtered by us. As a result, the yield of the target product 3a was found to be not greatly improved. In order to investigate the role of Pd in the reaction, we investigated the effect of Pd(OAC)2 (entry 13) and PdCl2 (entry 14) as catalysts on the reaction under the standard conditions. And it was found that the recyclable Pd nanoparticles acted as a catalyst to strengthen the reaction compared with the Pd salt catalyst. Pd/ZrO2 (3 wt%, 10 mol%) was used as photocatalyst in CH3CN under the exposure of an incandescent lamp at air conditions for 12 h which is the optimal conditions to produce the product 3a (3-thioazaspiro[4, 5]trienones). After that, the mass fraction ratio and content of the catalyst were investigated (Table 1). And only a small amount of the target product 3a was examined in the absence of visible light irradiation (entry 12) under the standard conditions.
|
|
Table 1 Optimization of the reaction conditions.a |
A key advantage of heterogeneous catalysis is the possibility of catalyst recovery. A series of spirocyclization reactions of N-methyl-N, 3-diphenylpropanamide 1a and thiophenol 2a were studied under visible light irradiation, demonstrating that Pd/ZrO2 is a recyclable catalyst. At the end of each reaction cycle, the Pd/ZrO2 catalyst was separated by a centrifuge and thoroughly washed twice with ethanol and dried for subsequent reaction. We have reused five times of Pd/ZrO2 catalyst to carry out the reaction under the same reaction conditions and obtained some research data. As shown in Fig. 2A, the Pd/ZrO2 catalyst was reused for several cycles without significantly exhibiting low and moderate activity.

|
Download:
|
| Fig. 2. (A) The photocatalytic activity of the Pd/ZrO2 catalyst after being recycled five times. (B) Light and dark reaction yields under different temperature conditions. (C) Dependence of the catalytic activity of Pd/ZrO2 photocatalyst for the spirocyclization reaction of the aromatic alkyne with the sulfhydryl group on the intensity of irradiation. The light contribution = [(Ylight – Ydark)/Ylight]× 100%, where Ylight and Ydark are the product yields under irradiation and controlled in the dark, respectively. | |
The products yields tend to be stable when the reaction temperature exceeds 30℃. And the conversion rate at different temperatures has been obtained by calculating the difference between the yields under visible light and the dark reaction yields, to found that this reaction system is more efficient, green and energy efficient at 30℃ (Fig. 2B).
We studied the relationship between photocatalytic activity and incident light intensity (irradiance). The spirocyclization reactions of the aromatic alkyne with the sulfhydryl group are carried out at different irradiances. The product yield is also correspondingly increased when the intensity of light is enhanced (Fig. 2C). The contribution of irradiation to the reaction is manifested by calculating the reaction conversion rate obtained by subtracting the reaction yield obtained in the dark under the same reaction conditions, and the yield of the product obtained in the dark is considered to be heat contribution.
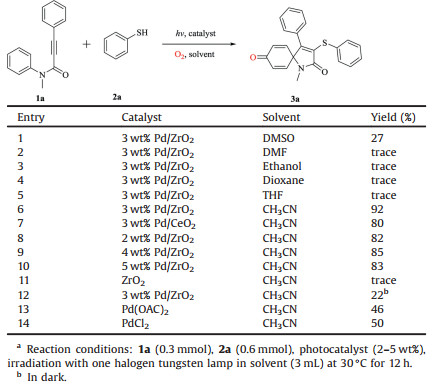
Based on the optimization research, some extended researchs on this new spirocyclization reaction, and the results are summarized in Scheme 2. First, whether the substituent on the thiophenol is an electron withdrawing group (F, Cl or Br) or an electron donating group (Me or MeO), the reaction proceeds well and has a good yield (3b-3k). Afterwards, we studied different substituents of the aniline moiety of N-arylpropiolamides, mainly the ortho and meta positions are compatible with this reaction, and a good yield can be obtained (3l-3o), The desired spirocyclization product can also be obtained by replacing N-Me with N-H, as is the substitution on the alkyl alkyne (3p).
|
Download:
|
| Scheme 2. Visible-light-induced alkynes and thiols to form 3-sulfenyl azaspiro[4, 5]trienonesa. | |
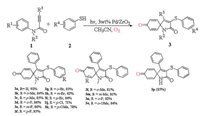
We conducted several experiments in order to investigate the reaction mechanism of this reaction and the source of oxygen in the product (Scheme 3). The results indicate that the oxygen in the product is derived from air rather than H2O (Scheme 3, a and b). Interestingly, we did not get the desired product when we added the group at the para position of the aniline which further confirmed that product 3 has a change in the para position (Scheme 3, c). Then we conducted several sets of experiments and discussed, no new products were produced in the respective independent reactions of 1a and 2a under the same conditions, this result indicates that the original reaction occurred due to the interaction of 1a and 2a. Moreover, a well-known radical trapping agent TEMPO (2, 2, 6, 6-tetramethyl-1-piperidinyloxy) can inhibited the occurrence of the reaction when it is added to the reaction system in other things being equal. And the TEMPO-trapped complex (PhS-TEMPO) was detected by Mass Spectrometry analysis (Scheme 3, d), which supports that ArS radical may involve in the reaction and promote reaction pathway to gain further insight the mechanism of this process.
|
Download:
|
| Scheme 3. Control experiments. | |
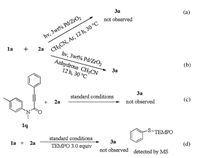
Through the investigation of the above experimental results and previous experimental reports [31-38], a possible mechanism was proposed as shown in Scheme 4 [39]. Initially, the high-energy electrons excited on the surface of the metal palladium nanoparticles enter the deductive band through the band transition to the surface, thereby entering the molecular anti-bond orbital under the irradiation of visible light. So the thiophenol adsorbs on the surface of the catalyst to form a complex [40]. Then, the electron holes are converted into each other and the electrons enter the hydrogen in the sulfhydryl group. Subsequently, the hydrogen of thiophenol is extracted by Pd/ZrO2 nanoparticles to form a radical 4 (ph-S) and the removed hydrogen is adsorbed on the surface of nano-palladium to form H-Pd NPs, which attacks the acetylenic bond of the substrate 1 to form a radical intermediate 5 (detected by MS in the Supporting information). Then, the intramolecular spirocyclization of the radical intermediate 5 with aromatic ring may result in a radical intermediate 6 which forms a free radical intermediate 7 under the oxidation of oxygen in the air while losing one H positive ion. Next the radical intermediate 7 is transferred to Pd/ZrO2 nanoparticles to form intermediate 8 (detected by MS in the Supporting information). After that, the 1/2 oxygen anion is removed to form a radical intermediate 9. Meanwhile, the oxygen anion reacts with the H positive ion to form water. Subsequently, the radical intermediate 9 forms the final product 3 and the activation site on the photocatalyst return to the state before starting the reaction (Scheme 4).
In conclusion, a simple, green and recyclable method for synthesizing various 3-sulfenyl azaspiro[4, 5]trienones can be driven in visible light irradiation at 30℃. During this photocatalytic process, Pd NPs loaded on ZrO2 contributes activation of the reactants and may reveal a new spirocyclic oxygenation pathway. This reaction pathway reveals a class of recyclable catalytic processes that utilize visible light for energy conservation, lower environmental impact for the synthesis of photocatalytic chemical. The novel synthetic method is expected to be synthesized in medicinal chemical synthesis with wide range of application prospects.
|
Download:
|
| Scheme 4. Plausible reaction pathway. | |
Acknowledgment
We are grateful for financial support from Jiangsu Planned Projects for Postdoctoral Research Funds (No. 2018K293C).
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.06.008.
| [1] |
J. Kobayashi, T. Kubota, Nat. Prod. Rep. 26 (2009) 936-962. DOI:10.1039/b813006j |
| [2] |
H.B. Park, Y.J. Kim, J.K. Lee, K.R. Lee, H.C. Kwon, Org. Lett. 14 (2012) 5002-5005. DOI:10.1021/ol302115z |
| [3] |
Y.L. Yang, F.R. Chang, Y.C. Wu, Helv. Chim. Acta 87 (2004) 1392-1399. DOI:10.1002/hlca.200490127 |
| [4] |
V.A. D'yakonov, O.A. Trapeznikova, A.D. Meijere, U.M. Dzhemilev, Chem. Rev. 114 (2014) 5775-5814. DOI:10.1021/cr400291c |
| [5] |
W.T. Wu, L. Zhang, S.L. You, Chem. Soc. Rev. 45 (2016) 1570-1580. DOI:10.1039/C5CS00356C |
| [6] |
W.H. Bao, C. Wu, W.M. He, Org. Biomol. Chem. 16 (2018) 8403-8407. DOI:10.1039/C8OB01820K |
| [7] |
L.Y. Xie, Y.J. Li, W.M. He, Green Chem. 19 (2017) 5642-5646. DOI:10.1039/C7GC02304A |
| [8] |
Q.S. Liu, W. Wei, Green Chem. 21 (2019) 1609-1613. DOI:10.1039/C9GC00222G |
| [9] |
W. Wei, H. Cui, D. Yang, H. Wang, Green Chem. 19 (2017) 5608-5613. DOI:10.1039/C7GC02330H |
| [10] |
H. Cui, W. Wei, D. Yang, H. Wang, RSC Adv. 5 (2015) 84657-84661. DOI:10.1039/C5RA16548B |
| [11] |
X.H. Yang, R.J. Song, Y. Li, B. Liu, J. Org. Chem. 79 (2014) 4582-4589. DOI:10.1021/jo5005982 |
| [12] |
W.T. Wang, J.H. Li, Org. Chem. Front. 1 (2014) 484-489. DOI:10.1039/c4qo00006d |
| [13] |
L.J. Wang, A.Q. Wang, Y. Xia, X.X. Wu, X.Y. Liu, Chem. Commun. 50 (2014) 13998-14001. DOI:10.1039/C4CC06923D |
| [14] |
L. Wu, F.L. Tan, M. Li, Org. Chem. Front. 4 (2017) 350-353. DOI:10.1039/C6QO00691D |
| [15] |
Y.Q. Yuan, P.S. Kumar, C.N. Zhang, M.H. Yang, S.R. Guo, Org. Biomol. Chem. 15 (2017) 7330-7338. DOI:10.1039/C7OB01552F |
| [16] |
X. Yang, X. Ouyang, W. Wei, J. Li, Adv. Syn. Catal. 46 (2015) 1161-1166. |
| [17] |
T. Tana, X.W. Guo, Chem. Commun. 52 (2016) 11567-11570. DOI:10.1039/C6CC05186C |
| [18] |
Q. Xiao, E. Jaatinen, H. Zhu, Chem. Asian J. 9 (2014) 3046-3064. DOI:10.1002/asia.201402310 |
| [19] |
D.P. Hari, B. Konig, Angew. Chem. Int. Ed. 52 (2013) 4734-4743. DOI:10.1002/anie.201210276 |
| [20] |
S. Ni, J. Cao, H. Mei, Y. Pan, Green Chem. 18 (2016) 3935-3939. DOI:10.1039/C6GC01027J |
| [21] |
S. Sarina, H. Zhu, E. Jaatinen, et al., J. Am. Chem. Soc. 135 (2013) 5793-5801. DOI:10.1021/ja400527a |
| [22] |
M. Hartings, Nature Chem. 4 (2012) 764. DOI:10.1038/nchem.1437 |
| [23] |
S.S. Stahl, Science 309 (2005) 1824-1826. DOI:10.1126/science.1114666 |
| [24] |
C.C. Johansson Seechurn, M.O. Kitching, T.J. Colacot, V. Snieckus, Angew. Chem. Int. Ed. 51 (2012) 5062-5085. DOI:10.1002/anie.201107017 |
| [25] |
T.Y. Shang, L.H. Lu, Z. Cao, et al., Chem. Commun. 55 (2019) 5408-5419. DOI:10.1039/C9CC01047E |
| [26] |
B.H. Zhang, C.Y. He, Chin. Chem. Lett. 28 (2017) 1751-1754. DOI:10.1016/j.cclet.2017.03.039 |
| [27] |
D.Q. Dong, Z.L. Wang, Chin. Chem. Lett. 28 (2017) 1597-1599. DOI:10.1016/j.cclet.2017.03.008 |
| [28] |
R.Z. Mao, X.S. Ye, Chin. Chem. Lett. 29 (2017) 61-64. |
| [29] |
Y.Q. Ge, P.H. Diao, C. Guo, Chin. Chem. Lett. 29 (2018) 903-906. DOI:10.1016/j.cclet.2018.01.002 |
| [30] |
L.H. Wang, X.L. Cui, Chin. Chem. Lett. 29 (2018) 907-910. DOI:10.1016/j.cclet.2018.05.007 |
| [31] |
W. Wei, C. Liu, D. Yang, et al., Chem. Commun. 49 (2013) 10239-10241. DOI:10.1039/c3cc45803b |
| [32] |
Q. Xiao, S. Sarina, H. Zhu, ACS Catal. 4 (2014) 1725-1734. DOI:10.1021/cs5000284 |
| [33] |
Q. Xiao, S. Sarina, J. Esa, H. Zhu, Green Chem. 16 (2014) 4272-4285. DOI:10.1039/C4GC00588K |
| [34] |
Q. Xiao, Z. Liu, A. Bo, et al., J. Am. Chem. Soc. 137 (2015) 1956-1966. DOI:10.1021/ja511619c |
| [35] |
S. Sarina, S. Bai, H. Zhu, Green Chem. 16 (2014) 331-341. DOI:10.1039/C3GC41866A |
| [36] |
J. Zhao, Z. Zheng, S. Bottle, et al., Chem. Commun. 49 (2013) 2676-2678. DOI:10.1039/c3cc38985e |
| [37] |
W. Cui, Q. Xiao, S. Sarina, Catal. Today 235 (2014) 152-159. DOI:10.1016/j.cattod.2014.04.015 |
| [38] |
Z. Xing, M. Yang, H. Sun, Green Chem. 20 (2018) 5117-5122. DOI:10.1039/C8GC02237B |
| [39] |
H.F. Qian, C.K. Li, J.P. Zou, Org. Lett. 20 (2018) 5947-5951. DOI:10.1021/acs.orglett.8b02639 |
| [40] |
Y. Zhang, L. Pei, Z.J. Zheng, Mater. Chem. A 3 (2015) 18045-18052. DOI:10.1039/C5TA03214H |
 2020, Vol. 31
2020, Vol. 31