To endow materials with certain functionality such as degradability, drug loading capability, responsiveness, targeting or self-assembly is what researchers have been pursuing [1-4]. Among the various functional systems, stimuli-responsive materials which are capable of response under physiological stimuli including changes in pH [5], temperature [6], the enzyme concentration [7], redox gradients [8] and so on, have attracted worldwide interest in the field of medicine, food, and environmental engineering. In this regard, disulfide-containing materials are most common reducing-responsive systems because the disulfide bonds are unstable to thiol-type reductants which usually shows a high discrepancy in concentration between intracellular and extracellular milieu [9, 10], and has become a hot topic in the last few years.
Polyurethane (PU), an important class of multi-purpose synthetic polymer, has been widely applied in bioengineering due to its easy synthesis, excellent biocompatibility and customizable molecular structures [11-13], and disulfide bonds have been incorporated into the hard segment of PUs by diols or diamines in the chain extension reaction process [14, 15]. As a kind of stimuli-responsive materials, the disulfide-containing PUs are widely investigated as drug delivery carriers, especially in cancer therapeutics [16-18], which are usually multiblock structures prepared via multi-step complex techniques and can finally self-assemble in an aqueous phase to form a micellar structure [10, 14, 16-19]. It is also reported that the introduction of disulfide bonds into the backbone of the carbohydrate-based PUs could provide the PU with degradability in the reducing environment [20-22]. On the other hand, the waterborne polyurethane (WPU), which has an ionic group to allow PU particles to be dispersed and stably stored in water, has received increasing interest in a broad range of applications due to its convenient synthesis, versatility and environmental friendliness [9, 23]. To the best of our knowledge, however, most of the investigations were only concentrated on the diversities of the micellar micro-morphology or micro-structure under reducing stimulation, and the reducing-responsive behavior of the macro-PU materials has not been reported.
In our previous work, a new kind of castor oil-based WPU nanoemulsions have been prepared and used as the carrier in the fabrication of nano-pesticide systems [24, 25]. In this study, a novel functional disulfide-containing WPU (DS-WPU) nanoemulsion was prepared via one-step in situ phase inverse emulsification. The chemical composition, the colloidal properties as well as the reducing-responsive property of the nanoemulsion was investigated, and the self-assembly behavior of DS-WPU in the reductant solution was intensively studied.
In experiments, all the chemicals were purchased from commercial suppliers and were used without further purification unless otherwise noted, including isophorone diisocyanate (IPDI, 3 A Chemicals), polyether diol (N220, Jining Baichuan Chem), dimethylolpropionic acid (DMPA, Beijing Linshi Chem), dibutyltin dilaurate (DBTDL, Tianjin Guangfu Fine Chem), 2-hydroxyethyl disulfide(HEDS, 90%, Aladdin Bio-Chem Technology), tetrahydrofuran (THF, Aladdin Bio-Chem Technology), triethylamine (TEA, Beijing Tongguang Fine Chem), acetone(Beijing Tongguang Fine Chem) and dithiothreitol (DTT, Shanghai Titan Scientific). Deionized water was used throughout.
The DS-WPU nanoemulsion was prepared via one-step in situ phase inverse emulsification technique as follows. IPDI (3.49 g), N220 (6.40 g) and DBTDL (2 drops) were first mixed in a 100 mL three-necked round-bottom flask with a reflux condenser and reacted at 80 ℃ with the stirring speed of 300 rpm for 2 h. DMPA (0.54 g) dissolved in acetone (1 mL) was added into the flask and the reaction continued for another 1 h. Then HEDS (0.62 g) and acetone (2 mL) were added into the reaction system. After a two-hour reaction, the system was cooled to room temperature, and the mixture of TEA (0.41 g) and acetone (2 mL) was added into the system, and the DS-WPU prepolymer was prepared by stirring for 10 min. Afterward, 24.7 g of water was charged into the system and in situ phase inversion emulsification of the DS-WPU prepolymer was conducted at 1000 rpm for 40 min. Ultimately, the nanoemulsion obtained by removing acetone with vacuum rotary evaporation.
Identification of the disulfide bonds and thiols in the sample of the latex film were qualitatively determined via Raman spectra (LabRAM HR800; Horiba Jobin Yvon) using a laser excitation wavelength of 633 nm.
Solid content (SC) of the DS-WPU nanoemulsion was determined thermo-gravimetrically. Hydrodynamic diameter (Dp), polydispersity index (PDI), and zeta potential (ζ) of the DS-WPU latex particles were measured with laser scattering method (Zetasizer 3000 HS; Malvern) at 25 ℃. Morphology of the dried latex particles was observed on a transmission electron microscope (TEM, H-7650B; Hitachi) with an accelerating voltage of 80 kV.
Reducing-responsive property of the DS-WPU latex film was evaluated as follows. About 100 mg of the latex film was immersed into 20 mL of 5 mg/mL DTT aqueous solution in a sealed bottle, and the bottle was then placed in a shaking incubator (HZ-9210 K; Hualida) at 25 ℃ with a shaking speed of 150 rpm. Meanwhile, as a controlled experiment, another 100 mg of the latex film was immersed into 20 mL of water and the bottle was treated in the same process as above. At specified time intervals, the Dp, TEM image, Raman spectra and molecular weights of the particles dispersed in DTT aqueous solution were characterized. The molecular weights were determined by a gel permeation chromatography (GPC, LC-20 A; Shimadzu) instrument with THF as the eluent (1 mL/min), in which the sample was obtained by freeze-drying the precipitate for 5 h which was collected from the centrifugationof DTT reducing emulsion with a speed of 12, 000 rpm.
The preparation process of DS-WPU nanoemulsion can be illustrated in Fig. S1 (Supporting information). The DS-WPU prepolymer with hydrophilic carboxyl groups was first synthesized by step-growth addition polymerization, and the prepolymer was then dispersed in water to form a stable emulsion via self-emulsification through vigorous mechanical stirring.
The resulted DS-WPU nanoemulsion can form a uniform latex film on the surface of other materials. In addition to the usage of forming the latex film, it also offers a possibility of helping the hydrophobic compounds especially the anti-cancer drugsdisperse stably in water as a carrier [9, 21, 22].
As Raman spectra (Fig. S2 in Supporting information) indicated, the stretching vibration of S-S group in the DS-WPU was at 512 cm−1 while there were no peaks in the same position in the spectrum of WPU, indicating that disulfide bonds were incorporated into the DS- WPU system successfully.
Colloidal property of the nanoemulsion is important for its application. As listed in Table S1 (Supporting information), Dp of the DS-WPU nanoemulsion was about 30 nm with a narrow size distribution, which was in accord with the TEM image (Fig. S3 in Supporting information). The nanoscale and uniform spherical morphology of the DS-WPU nanoemulsion was not only beneficial to form a smooth latex film, but also allow an increased circulation time and enhanced permeability in vivo when used as the drug carrier [26].
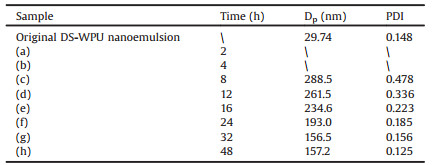
To prove the responsiveness of the DS-WPU system, the reducing-responsive experiment was conducted and the results were displayed in Fig. 1 and Table 1. As indicated, the clear DTT aqueous solution turned to a milky emulsion gradually and the latex film completely disintegrated at last (Figs. 1a–f) in the reducing process, while the aqueous phase kept clear in the controlled experiment (Fig. S4 in Supporting information). Note that, with the increase of reducing time, the Dp of the self-assembled particles decreased from 288 nm to around 157 nm and finally remained constant, which was much greater than that of the original DS-WPU emulsion, and the size distribution became narrower. These results clearly suggested that the DS-WPU had great responsiveness under the reducing environment and a self-assembly behavior of WPU molecular chains existed in the DTT aqueous solution.

|
Download:
|
| Fig. 1. The change of the DS-WPU latex film in 5 mg/mL DTT aqueous solution versus the reducing time: (a) 2 h; (b) 4 h; (c) 8 h; (d) 12 h; (e) 16 h; (f) 24 h; (g) 32 h; (h) 48 h. | |
|
|
Table 1 Colloidal properties of the self-assembled emulsions obtained at different reducing time. |
In order to further understand this self-assembly behavior, Raman spectra and GPC of the self-assembled emulsion particles were conducted, and a possible self-assembly mechanism was described in Fig. 2. The disulfide bonds in the DS-WPU latex film random cleaved gradually under reducing environment, and resultant amphiphilic polymer chains first dissolved in the DTT aqueous solution and when the concentration reached to its CMC (critical micelle concentration), they would self-assemble into big spherical particles to form an emulsion. With time increasing, the long polymer chains with numerous disulfide bonds would continue to be cleaved into short chains which would self-assemble to form small particles, resulting in a decrease of particles sizes. Before reaching equilibrium, the existence of big particles led to a relatively large size distribution which conformed to the result of the TEM image in Fig. 2. The Raman spectra and GPC results supported this perspective. The Raman spectra (Fig. 3a) suggested that the disappearance of disulfide bonds (512 cm−1) and the appearance of thiols (2570 cm−1) in the WPU system with the increase of the reducing time, meaning that the thiol-disulfide exchange reactions occurred. Moreover, the Mn (Fig. 3b) of the DS-WPU particles in the self-assembled emulsion decreased from 53, 896 to 15, 400 with the reducing time increased from 8 h to 48 h, which clearly indicated the breakages of the polymer chains.

|
Download:
|
| Fig. 2. Self-assembly mechanism of the DS-WPU chains in the DTT aqueous solution. | |

|
Download:
|
| Fig. 3. (a) Raman spectra and (b) GPC curves of the self-assembled polymer particles obtained at different reducing time. | |
It is worth pointing out that the final self-assembled emulsion could re-form a latex film by drying with little difference in appearance from the previous latex film.
In summary, the DS-WPU nanoemulsion was prepared by one-step in situ phase inverse emulsification technique which was facile and economic. The size of DS-WPU emulsion particles was nanoscale and could form a uniform latex film. Moreover, the DS-WPU system shown great responsiveness under the reducing environment, in which a self-assembly behavior along with a long-to-short process of polymer chains was observed, and the self-assembled emulsion which had a much greater particle size than that of the original DS-WPU nanoemulsion could also re-form a latex film. This novel functional polyurethane nanoemulsion has a great potential to be further explored and follow-up work is still in progress.
AcknowledgmentsThe authors gratefully acknowledge the National Key Research and Development Program of China (No. 2017YFD0200704) and the National Basic Research Program of China (973 Program, No. 2014CB932202) for the financial supports.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.015.
| [1] |
M. Ding, J. Li, X. He, et al., Adv. Mater. 24 (2012) 3639-3645. DOI:10.1002/adma.201200954 |
| [2] |
W. Qu, Y. Xia, L. Jiang, L. Zhang, Z. Hou, Chin. Chem. Lett. 27 (2016) 135-138. DOI:10.1016/j.cclet.2015.07.018 |
| [3] |
X. Zhou, L. Li, H. Qin, et al., RSC Adv. 8 (2018) 21613-21620. DOI:10.1039/C8RA03374A |
| [4] |
J. Fu, M. Zhang, L. Jin, et al., Appl. Surf. Sci. 470 (2019) 543-554. DOI:10.1016/j.apsusc.2018.11.168 |
| [5] |
Y. Jin, L. Song, Y. Su, et al., Biomacromolecules 12 (2011) 3460-3468. DOI:10.1021/bm200956u |
| [6] |
H. Wei, S. Cheng, X. Zhang, R. Zhuo, Prog. Polym. Sci. 34 (2009) 893-910. DOI:10.1016/j.progpolymsci.2009.05.002 |
| [7] |
L. Dong, S. Xia, K. Wu, et al., Biomaterials 31 (2010) 6309-6316. DOI:10.1016/j.biomaterials.2010.04.049 |
| [8] |
Y. You, C. Hong, C. Pan, Macromolecules 42 (2009) 573-575. DOI:10.1021/ma802403w |
| [9] |
I. Omrani, N. Babanejad, H.K. Shendi, M.R. Nabid, Mater. Sci. Eng. C 70 (2017) 607-616. DOI:10.1016/j.msec.2016.09.036 |
| [10] |
R. Tong, H. Xia, X. Lu, J. Mater. Chem. B 1 (2013) 886-894. DOI:10.1039/C2TB00222A |
| [11] |
M. Ding, J. Li, H. Tan, Q. Fu, Soft Matter. 8 (2012) 5414-5428. DOI:10.1039/c2sm07402h |
| [12] |
S. Grad, L. Kupcsik, K. Gorna, S. Gogolewski, M. Alini, Biomaterials 24 (2003) 5163-5171. DOI:10.1016/S0142-9612(03)00462-9 |
| [13] |
C. Zhang, X. Wen, N.R. Vyavahare, T. Boland, Biomaterials 29 (2008) 3781-3791. DOI:10.1016/j.biomaterials.2008.06.009 |
| [14] |
Y. Yao, H. Xu, C. Liu, et al., RSC Adv. 6 (2016) 9082-9089. DOI:10.1039/C5RA24903A |
| [15] |
X. Xing, R. Lai, P. Dong, J. Luo, Acta Polym. Sin. 5 (2014) 678-685. |
| [16] |
S. Yu, C. He, J. Ding, et al., Soft Matter. 9 (2013) 2637-2645. DOI:10.1039/c2sm27616j |
| [17] |
X. He, M. Ding, J. Li, et al., RSC Adv. 4 (2014) 24736-24746. DOI:10.1039/C4RA01478B |
| [18] |
N. Song, M. Ding, Z. Pan, et al., Biomacromolecules 14 (2013) 4407-4419. DOI:10.1021/bm401342t |
| [19] |
M. Ding, X. Zeng, X. He, et al., Biomacromolecules 15 (2014) 2896-2906. DOI:10.1021/bm500506v |
| [20] |
L. Romero-Azogil, E. Benito, M.G. García-Martín, J.A. Galbis, Eur. Polym. J. 94 (2017) 259-269. DOI:10.1016/j.eurpolymj.2017.07.012 |
| [21] |
M. Violante de Paz, F. Zamora, B. Begines, C. Ferris, J.A. Galbis, Biomacromolecules 11 (2010) 269-276. DOI:10.1021/bm9011216 |
| [22] |
C. Ferris, M. Violante de Paz, Á. Aguilar-de-Leyva, I. Caraballo, J.A. Galbis, Polym. Chem. 5 (2014) 2370-2381. DOI:10.1039/c3py01572f |
| [23] |
M. Jiang, Z. Zheng, X. Ding, X. Cheng, Y. Peng, Colloid Polym. Sci. 285 (2007) 1049-1054. DOI:10.1007/s00396-007-1658-0 |
| [24] |
H. Qin, H. Zhang, L. Li, et al., RSC Adv. 7 (2017) 52684-52693. DOI:10.1039/C7RA10640H |
| [25] |
H. Zhang, H. Qin, L. Li, et al., J. Agric. Food Chem. 66 (2018) 6552-6560. DOI:10.1021/acs.jafc.7b01401 |
| [26] |
R. Kinoshita, Y. Ishima, M. Ikeda, et al., J. Control. Release 217 (2015) 1-9. DOI:10.1016/j.jconrel.2015.08.036 |
 2020, Vol. 31
2020, Vol. 31