b The Molecular Foundry, LBNL, Berkeley, CA 94702, United States;
c aBeam Technologies, Castro Valley, CA 94546, United States
High refractive index polymeric optical materials are increasingly attracting attention both in academic research and industrial fields due to their characteristics related to lightweight, easy processing and outstanding mechanical properties. Polymers with high index of refraction have potential applications in advanced optical devices [1-6] including organic light-emitting diodes [7-10], microlenses [11-14], image sensors [15, 16], photonic crystals [11, 17-24] and matamaterials [25, 26]. According to the classical Lorentz–Lorenz equation, the refractive index of a polymer can be reliably predicted based on the molar refractions and molar volumes of its substituent groups and backbone repeating units [27-29]. Therefore, incorporating chemical groups with high molar refraction and low molar volume is a common strategy used to considerably enhance the refractive index of polymers. Generally speaking, the introduction of aromatic groups, heavy halogens (except fluorine), sulfur atoms, and metal atoms with high molar refractions can effectively improve the refractive index of polymers.
Sulfur is one of the elements most commonly used for increasing a polymer's refractive index due to its high polarizability, stability, and straightforward insertion in its molecular structure. In recent years, various high refractive index polymers containing sulfur have been synthesized for advanced optical applications including poly(phenylene thioether)s [13, 30-33], polyimides [34, 35], poly(thioether sulfone)s [36-38] and poly(phenyl quinoxaline)s [39, 40]. Unfortunately, these sulfur-containing polymers with high refractive index were conceived following a complex and elaborated synthesis which along with their poor processability, inhibit their large scale production. They also showed low transparency which makes them unsuitable for application in optical devices where thick films are required.
Acrylate polymers have been extensively used in a broad variety of fields owing to their facile synthesis, excellent optical transparency, convenient processability and great mechanical properties [41-46]. Most of the existing acrylate polymers exhibit low refractive index (~1.50), which considerably prevents their prospective application in advanced optical devices [47]. Few reports can be found with acrylic polymers having a higher refractive index (over 1.60) including poly(pentabromophenyl methacrylate), poly(2, 4, 6-tribromophenyl methacrylate) and poly(2-((1, 3-dithiolan-2-yl)methylthio)ethylmethacrylate derivative) [48-54]. However, the halogen presence in their chemical structures restricts their use due to the known adverse effect to the environment [55]. Designing high refractive index acrylates with excellent transparency in the visible range and a convenient and cost effective synthetic method is of great significance for novel optical applications. High thermal stability of the polymers is another desired property to withstand the high processing and environmental conditions the polymers are exposed to during device integration and service life.
In this report, three novel acrylate polymers with high index of refractioncontaining sulfur atoms and rigid aromatic rings are presented. They show excellent optical transparency in the visible wavelength range along with high thermal resistance. The developed acrylates were successfully nanopatterned with high resolution and fidelity using nanoimprint lithography. Three monomershaving different chemical structure were synthesized and chemically reacted at low temperatures to generate the high index acrylate monomers (Scheme 1). The chemical structures of the synthesized acrylate monomers were determined by Fourier transform infrared (FT-IR) and nuclear magnetic resonance (NMR) spectroscopic analyses. The acrylate monomers are polymerized by a free radical polymerization to produce high refractive index crosslinked polymer films. The transmittance of 30-μm-thick polymer films fabricated by melt polymerization at a temperature of 140 ℃ for 2 h was measured with UV–vis spectroscopy. Thermogravimetric analysis (TGA) of these polymers shows a thermal stability higher than 250 ℃. The presented acrylates show excellent optical properties and embossability and are promising candidates for a wide range of applications in optical and optoelectronic devices.
|
Download:
|
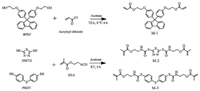
| Scheme 1. Synthetic routes of M-1, M-2 and M-3. | |
The refractive index is one of the most important optical properties of organic optical materials and is directly related to the chemical structure, electrical and magnetic properties. Therefore, a good understanding of the structure-property relationships of optical polymers is essential for designing high refractive index materials with improved properties and applications. According to the Lorentz–Lorenz equation, which defines the relationship between the refractive index and the chemical structure of polymers, the introduction of sulfur atoms and aromatic rings is useful for achieving high refractive index materials.
In this research, three acrylate monomers (M-1, M-2 and M-3) containing different chemical structures (fluorenyl, thiadiazole and diphenyl thioether group) were synthesized in high yield through a facile one-step procedure; the synthetic procedure of these acrylate monomers is depicted in Scheme 1. The monomer M-1 was synthesized by the typical nucleophilic substitution reaction of bisphenoxyethanolfluorene (BPEF) and acryloyl chloride. M-2 and M-3 were synthesized by reacting 2, 5-dimercapto-1, 3, 4-thiadiazole (DMTD) and phenoxybenzene-4, 4′-dithiol (PBDT) with 2-isocyanatoethyl methacrylate (IEM) by nucleophile addition reaction. The chemical structures of these synthetic monomers were determined by using FT-IR and NMR spectroscopic analyses.
The FT-IR spectra of the synthesized monomers are shown in Fig. 1. In the spectrum of M-1, the characteristic absorptions of the acrylate group related to the C=O stretching vibration at 1728 cm-1, C=C stretching vibration at 1638 cm-1, and C–H in-plane rotating vibration 810 cm-1 reveal that the acrylate double bond has been incorporated into M-1 successfully. As for M-2 and M-3, apart from the characteristic peaks of the methacrylate double bond (C=C stretching vibration at 1638 cm-1 and C–H in-plane rotating vibration 810 cm-1), their FT-IR spectra curves also exhibit the characteristic vibration absorptions of the urethane carbonyl groupaccording to the N-H stretching vibration at 3427 and 3272 cm-1 and C=O stretching vibration at 1716 cm-1, which indicate that both the urethane carbonyl and methacrylate moieties have been incorporated into M-2 and M-3.

|
Download:
|
| Fig. 1. FT-IR spectra (left) of and 1H NMR spectra (right) of M-1, M-2 and M-3 monomers. | |
In order to further validate the chemical structures of the synthetic monomers, 1H and 13C NMR spectroscopies were performed on a 400 MHz NMR instrument. Fig. 1 shows the 1H NMR spectra of three synthetic monomers. As shown in Fig. 1, there are four groups of protons' signals at 4.24~4.48, 5.91~6.36, 6.68~7.14 and 7.32~7.89 ppm, which can be ascribed to the protons of methylene, acrylate double bond, phenyl and fluorenyl, respectively. Furthermore, all the characteristic peaks of carbons corresponding to M-1 are observed in the 13C NMR spectrum shown in Fig. S1 (Supporting information). Therefore, the monomer M-1 containing a fluorenyl structure was successfully synthesized by a nucleophilic substitution reaction. Similarly, M-2 and M-3 were characterized by 1H NMR (Fig. 1) and 13C NMR (Figs. S2 and S3 in Supporting information) spectroscopies and elemental analysis. The corresponding NMR characterization shows the molecular structures were satisfactory results corresponding obtained. The desired structures of both M-2 and M-3 were incorporated successfully via nucleophile addition reaction.
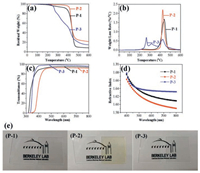
The melt polymerization of the synthetic monomers M-1, M-2 and M-3 was performed by a free radical process in a vacuum oven at 140 ℃ for 2 h, resulting in the corresponding optical transparent crosslinked polymers, P-1, P-2 and P-3. The thermal decomposition of the synthesized polymers was determined by TGA measurements (Figs. 2a and b). Temperature at 5 wt% weight loss (T5%), temperature at maximum weight loss rate (Tp) and char residue at 600 ℃ of the developed polymers are summarized in Table S1 (Supporting information). P-2 polymer exhibits excellent thermal stability, corresponding to a T5% of 402 ℃ which is high when compared with common acrylic polymers, such as a commercial poly(methyl methacrylate) (PMMA) [56]. The anaerobic char yield for this polymer is 21.78% at 600 ℃. The high thermal stability of P-2 polymer may be related to the presence of a strong polar thiadiazole ring structure, which can increase the intermolecular interaction by the formation of the hydrogen bonding. The thermal stability of P-3 is far below P-1 and P-2 and shows a T5% of 254 ℃ which is associated to its flexible thioether linkages providing a relatively low thermal property. The derivative thermogravimetric (DTG) curve of P-1 and P-2 exhibits a single peak which indicates their degradation occurs in a single step. However, the DTG curve of P-3 shows multiple peaks indicating its multistep degradation process.
|
Download:
|
| Fig. 2. Thermogravimetric analysis (a) and derivative thermogravimetric (b) curves of acrylatepolymers. Optical properties of acrylate polymers: (c) UV–vis transmittance spectra (film thickness: 30 μm) and (d) refractive index curves measured by ellipsometry. (e) Photographs of a bare piece of glass (left) and acrylate polymer film with thickness of 30 μm on glass (right). | |
The transmittance of 30-μm-thick polymer films fabricated by melt polymerization at a temperature of 140 ℃ for 2 h was measured with UV–vis spectroscopy. The transmittance, measured with UV–vis spectrophotometer, is shown in Fig. 2c. The color, cutoff wavelength (λcutoff) and transmittance of the proposed polymers are summarized in Table S2 (Supporting information). These results indicate that all the polymers present high transmittance values (≥90%) at the wavelength of 450 nm. The transparency can be mainly associated to the non-planar and bent structures of acrylate polymers, which leads to the inhibition of intermolecular packing. However, all polymers also show a different absorption behavior in the wavelength region from 300 nm to 450 nm (Fig. 2c), which can be a consequence of the existence of conjugated fluorenyl, thiadiazole and diphenyl thioether structures in these polymers. As we can observe in the photographs presented in Fig. 2e, except for the pale-yellow color P-2 film, all polymers exhibit excellent transparency when compared with the control samples, which are in good agreement with the results of the UV–vis transmittance spectra presented previously.
The refractive indices of the synthesized polymers were measured by ellipsometryto determine their possible applications in advanced optical devices and are shown in Fig. 2d. The refractive indices of P-1, P-2 and P-3 were measured as 1.6268, 1.6129 and 1.6363, respectively, at the wavelength of 550 nm (Table S2). These high values can be attributed to the high molar refraction provided by the presence of the aromatic groups and sulfur atoms in the main polymer chain.
The developed materials were nanopatterned with thermal nanoimprint lithography (Fig. S4 in Supporting information) using molds having different geometries and dimensions to demonstrate their capabilities as high refractive index films. These patterns include gratings with linewidths of 60 and 216 nm and photonic crystal structures with diameters of 150, 200, 280 and 320 nm. The high refractive index materials were imprinted with good fidelity and low defectivity as shown in Fig. 3. Acrylic polymers have been previously used for nanoimprinting, including PMMA, which has shown sub-10 nm resolution [57-59]. However, their low refractive index (the index of refraction of PMMA is 1.49 at 590 nm), exclude them from applications in advance optical devices. The nanoimprint results shown here demonstrate that the developed acrylate optical polymers have suitable properties for high resolution nanopatterning.

|
Download:
|
| Fig. 3. SEM images of nanoimprinted polymer patterns with various feature sizes: (a–d) P-1, (e–h) P-2 and (i–l) P-3. Magnification images are inserted in the upright corner of the SEM images. | |
In summary, three novel acrylate monomers containing different chemical structures were successfully synthesized by a facile one-step approach. Subsequently, the monomers were polymerized by free radical melt polymerization to produce thick crosslinked films polymers. The refractive index values of the crosslinked films laid in the range between 1.613 and 1.636, measured at a wavelength of 550 nm, due to the contribution of sulfur and aromatic groups. All of the resulting polymers exhibited optical transmittance above 90% in the visible wavelength range. The thermal stability of the developed polymers was higher than 250 ℃ as determined by TGA measurements. The synthesized acrylate monomers can be nanoimprinted with high fidelity and low defectivity to fabricate nanoscale gratings and photonic crystal various feature sizes. These results show the developed materials are promising for a broad range of optical applications.
AcknowledgmentsThis work was supported by the Molecular Foundry, Lawrence Berkeley National Laboratory, which is supported by the Office of Science and Office of Basic Energy Sciences of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. This study is also supported by National Natural Science Foundation of China (No. 51573011), Natural Foundation of Jiangsu Province (No. BK20150272) and Beijing Laboratory of Biomedical Materials. The first author acknowledges the scholarship support from the program of the China Scholarship Council (No. 201706880022) for study at Lawrence Berkeley National Laboratory.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.012.
| [1] |
C. Wang, T.M. Goldman, B.T. Worrell, et al., Mater. Horiz. 5 (2018) 1042-1046. DOI:10.1039/C8MH00724A |
| [2] |
T.S. Kleine, N.A. Nguyen, L.E. Anderson, et al., ACSMacro Lett. 5 (2016) 1152-1156. DOI:10.1021/acsmacrolett.6b00602 |
| [3] |
L. Wu, J. He, W. Shang, et al., Adv. Opt. Mater. 4 (2016) 195-224. DOI:10.1002/adom.201500428 |
| [4] |
S. Köber, M. Salvador, K. Meerholz, Adv. Mater. 23 (2011) 4725-4763. DOI:10.1002/adma.201100436 |
| [5] |
Y. Gan, X. Jiang, J. Yin, J. Mater. Chem. C 2 (2014) 5533-5539. DOI:10.1039/C4TC00350K |
| [6] |
C. Lü, B. Yang, J. Mater. Chem. 19 (2009) 2884-2901. DOI:10.1039/b816254a |
| [7] |
Q. Wei, R. Pötzsch, X. Liu, et al., Adv. Funct. Mater. 26 (2016) 2545-2553. DOI:10.1002/adfm.201504914 |
| [8] |
E. Kim, H. Cho, K. Kim, et al., Adv. Mater. 27 (2015) 1624-1631. DOI:10.1002/adma.201404862 |
| [9] |
T.W. Koh, J.A. Spechler, K.M. Lee, et al., ACS Photonics 2 (2015) 1366-1372. DOI:10.1021/acsphotonics.5b00346 |
| [10] |
Y.H. Kim, J. Lee, W.M. Kim, et al., Adv. Funct. Mater. 24 (2014) 2553-2559. DOI:10.1002/adfm.201303401 |
| [11] |
Y. Tang, C. Pina-Hernandez, Q. Niu, et al., J. Mater. Chem. C 6 (2018) 8823-8831. DOI:10.1039/C8TC02029A |
| [12] |
K. Nakabayashi, T. Imai, M.C. Fu, et al., Macromolecules 49 (2016) 5849-5856. DOI:10.1021/acs.macromol.6b01182 |
| [13] |
E.K. Macdonald, M.P. Shaver, Polym. Int. 64 (2015) 6-14. DOI:10.1002/pi.4821 |
| [14] |
J.G. Liu, M. Ueda, J. Mater. Chem. 19 (2009) 8907-8919. DOI:10.1039/b909690f |
| [15] |
H.Y. Ma, T.L. Wang, P.Y. Chang, et al., Nanomaterials 6 (2016) 44. DOI:10.3390/nano6030044 |
| [16] |
R.A. Potyrailo, V.M. Miesky, Chem. Rev. 108 (2008) 770-813. DOI:10.1021/cr068127f |
| [17] |
C. Pina-Hernandez, A. Koshelev, S. Dhuey, et al., Sci. Rep. 7 (2017) 17645. DOI:10.1038/s41598-017-17732-0 |
| [18] |
G. Calafiore, Q. Fillot, S. Dhuey, et al., Nanotechnology 27 (2016) 115303. DOI:10.1088/0957-4484/27/11/115303 |
| [19] |
A. Koshelev, G. Calafiore, C. Pina-Hernandez, et al., Opt. Lett. 41 (2016) 3423-3426. DOI:10.1364/OL.41.003423 |
| [20] |
C. Adachi, J. Appl. Phys. 53 (2014) 060101. DOI:10.7567/JJAP.53.060101 |
| [21] |
L. Li, H. Lin, S. Qiao, et al., Nat. Photonics 8 (2014) 643-649. DOI:10.1038/nphoton.2014.138 |
| [22] |
C. Pina-Hernandez, A. Koshelev, L. Digianantonio, et al., Nanotechnology 25 (2014) 325302. DOI:10.1088/0957-4484/25/32/325302 |
| [23] |
R. Betancur, P. Romero-Gomez, A. Martinez-Otero, et al., Nat. Photonics 7 (2013) 995. DOI:10.1038/nphoton.2013.276 |
| [24] |
C. Pina-Hernandez, V. Lacatena, G. Calafiore, et al., Nanotechnology 24 (2013) 065301. DOI:10.1088/0957-4484/24/6/065301 |
| [25] |
J.Y. Kim, H. Kim, B.H. Kim, et al., Nat. Commun. 7 (2016) 12911. DOI:10.1038/ncomms12911 |
| [26] |
T.P. Bigioni, X.M. Lin, T.T. Nguyen, et al., Nat. Mater. 5 (2006) 265-270. DOI:10.1038/nmat1611 |
| [27] |
C.J. Yang, S.A. Jenekhe, Chem. Mater. 7 (1995) 1276-1285. DOI:10.1021/cm00055a002 |
| [28] |
L. Lorenz, Ann. Phys. 247 (1880) 70-105. DOI:10.1002/andp.18802470905 |
| [29] |
H.A. Lorentz, Ann. Phys. 245 (1880) 641-665. DOI:10.1002/andp.18802450406 |
| [30] |
Y. Nakagawa, Y. Suzuki, T. Higashihara, et al., Polym. Chem. 3 (2012) 2531-2536. DOI:10.1039/c2py20325a |
| [31] |
Y. Nakagawa, Y. Suzuki, T. Higashihara, et al., Macromolecules 44 (2011) 9180-9186. DOI:10.1021/ma2020003 |
| [32] |
Y. Suzuki, K. Murakami, S. Ando, et al., J. Mater. Chem. 21 (2011) 15727-15731. DOI:10.1039/c1jm12402a |
| [33] |
N.H. You, T. Higashihara, Y. Oishi, et al., Macromolecules 43 (2010) 4613-4615. DOI:10.1021/ma100448d |
| [34] |
J.G. Liu, Y. Nakamura, Y. Suzuki, et al., Macromolecules 40 (2007) 7902-7909. DOI:10.1021/ma0713714 |
| [35] |
J.G. Liu, Y. Nakamura, Y. Shibasaki, et al., Macromolecules 40 (2007) 4614-4620. DOI:10.1021/ma070706e |
| [36] |
Y. Suzuki, T. Higashihara, S. Ando, et al., Macromolecules 45 (2012) 3402-3408. DOI:10.1021/ma300379w |
| [37] |
Y. Suzuki, T. Higashihara, S. Ando, et al., Polym. J. 41 (2009) 860-865. DOI:10.1295/polymj.PJ2009124 |
| [38] |
R. Okutsu, Y. Suzuki, S. Ando, et al., Macromolecules 41 (2008) 6165-6168. DOI:10.1021/ma800797p |
| [39] |
L.M. Goldenberg, V. Lisinetskii, Y. Gritsai, et al., Opt. Mater. Exp. 2 (2012) 11-19. DOI:10.1364/OME.2.000011 |
| [40] |
C. Li, Z. Li, J. Liu, et al., Polymer 51 (2010) 3851-3858. DOI:10.1016/j.polymer.2010.06.035 |
| [41] |
M.A.G. Lazo, M. Blank, Y. Leterrier, et al., Polymer 54 (2013) 6177-6183. DOI:10.1016/j.polymer.2013.09.004 |
| [42] |
J.D. Cho, S.S. Lee, S.C. Park, et al., Polym. Sci 130 (2013) 3098-3104. DOI:10.1002/app.39558 |
| [43] |
B.S. Kim, Bae Je, S. Lee, et al., Polym. Bull. 68 (2012) 2097-2105. DOI:10.1007/s00289-011-0668-8 |
| [44] |
N.H. You, T. Higashihara, S. Ando, et al., J. Polym. Sci. Part A: Polym. Chem. 48 (2010) 2604-2609. |
| [45] |
M. Nogi, S. Iwamoto, A.N. Nakagaito, et al., Adv. Mater. 21 (2009) 1595-1598. DOI:10.1002/adma.200803174 |
| [46] |
C.Y. Gao, B. Yang, J.C. Shen, J. Appl. Polym. Sci. 75 (2000) 1474-1479. DOI:10.1002/(SICI)1097-4628(20000321)75:12<1474::AID-APP5>3.0.CO;2-1 |
| [47] |
J. Zarian, J.A. Robbins. Patent US5298327A 1994.
|
| [48] |
Y. Tojo, Y. Arakwa, J. Watanabe, et al., Polym. Chem. 4 (2013) 3807-3812. DOI:10.1039/c3py00377a |
| [49] |
M. Maheswara, S.H. Oh, J.J. Ju, et al., Polym. J. 42 (2010) 249-255. DOI:10.1038/pj.2009.328 |
| [50] |
A. Nebioglu, J.A. Leon, I.V. Khudyakov, Ind. Eng. Chem. Res. 47 (2008) 2155-2159. DOI:10.1021/ie071443f |
| [51] |
R. Okutsu, S. Ando, M. Ueda, Chem. Mater. 20 (2008) 4017-4023. DOI:10.1021/cm800432p |
| [52] |
J.E. McGrath, L. Rasmussen, A.R. Shultz, et al., Polymer 47 (2006) 4042-4057. DOI:10.1016/j.polymer.2006.02.030 |
| [53] |
T. Matsuda, Y. Funae, M. Yoshida, et al., J. Appl. Polym. Sci. 76 (2000) 50-54. DOI:10.1002/(SICI)1097-4628(20000404)76:1<50::AID-APP7>3.0.CO;2-X |
| [54] |
R.A. Minns, R.A. Gaudiana, J. Macromol. Sci. Part A: Pure Appl. Chem. 29 (1992) 19-30. DOI:10.1080/10101329208054104 |
| [55] |
E. Goosey, Circuit World 32 (2006) 32-35. DOI:10.1108/03056120610683603 |
| [56] |
T. Ramanathan, S. Stankovich, D. Dikin, et al., J. Polym. Sci. Part B: Polym. Phys. 45 (2007) 2097-2112. DOI:10.1002/polb.21187 |
| [57] |
S.Y. Chou, P.R. Krauss, W. Zhang, et al., J. Vac. Sci. Technol. B 15 (1997) 2897-2904. DOI:10.1116/1.589752 |
| [58] |
S.Y. Chou, P.R. Krauss, P.J. Renstrom, Science 272 (1996) 85-87. DOI:10.1126/science.272.5258.85 |
| [59] |
S.Y. Chou, P.R. Krauss, P.J. Renstrom, Appl. Phys. Lett. 67 (1995) 3114-3116. DOI:10.1063/1.114851 |
 2020, Vol. 31
2020, Vol. 31 
