b University of Chinese Academy of Sciences, Beijing 100049, China;
c Institute of Polymer Science and Engineering, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
One-dimensional (1D) nanomaterials are promising in collection of chemical spills and heavy metal ions. As an example, carbon nanofibers are capable to absorb organic solvents [1, 2] and metal ions [3]. It is important to develop methods to prepare nanofibers with tunable composition and microstructure. Electro-spinning is effective to prepare one-dimensional nanofibers with tunable composition [4]. The nanofibers are easily broken in organic solvents [5, 6], which are usually thick above sub-micrometers [7, 8]. Especially, the yield is rather low. We have previously reported a simple method to large scale synthesize crosslinked PDVB nanofibers by precipitated cationic polymerization [9]. The nanofibers are stable against solvents, thus serving as effective gelators to immobilize almost all organic solvents. Chemical spills can be easily removed with the nanofibers. The PDVB nanofibers possess abundant reactive groups [10], making further modification [11] easier such as conjugation of ligands for effective absorption of heavy metal ions. Although the PDVB nanofibers are promising for environment remedy, small fragments are usually left in water after salvaged from water. It is highly required to completely remove the gelled chemical spills in an easy way.
Herein, we report the synthesis of magnetic responsive PDVB composite nanofibersin the presence of paramagnetic NPs [12] during the cationic polymerization(Supporting information). The NPs are encapsulated within the PDVB thus forming aggregates. The nanofibers are connected into a network. The magnetic responsive PDVB nanofibers make it easy to separate the gelled chemical spills with a magnet.
A PDVB nanofiber about 150 nm in diameter [9] was synthesized by the precipitated cationic polymerization in the n-hexane (Fig. 1a). The nanofiber is bamboo like structure with segmental hollow tubes compartmentalized (Fig. 1b). In the presence of the oleic acid capped Fe3O4 NP (Supporting information) during the polymerization, magnetic responsive PDVB nanofiber composites were achieved. At small amount of Fe3O4 NP for example 0.01 wt%, the PDVB nanofiber was achieved (Fig. 1c). A minority amount of irregular aggregates coexist. Moreover, some thin nanofibers ~50 nm in diameter are grown from the aggregates. The thick nanofibers are wrapped with the thin nanofibers forming an interconnected network. Growth of the thin nanofibers from the aggregates is confirmed by TEM observation (Fig. 1d). Many Fe3O4 NPs are encapsulated within the aggregates. At a high amount of the Fe3O4 NP for example 0.05 wt%, amount and length of the fine PDVB nanofibers are greatly increased (Fig. 1e). The fine nanofibers are interwoven forming a robust network with the aggregates encapsulated (Fig. S1a in Supporting information). At an extremely high amount of the Fe3O4 NP such as 0.2 wt%, nanoparticles of 30 nm in diameter are coagulated into irregular dendrites (Fig. 1f). TEM image indicates that each nanoparticle contains Fe3O4 NPs encapsulated with PDVB at the exterior surface (Fig. S1b in Supporting information).
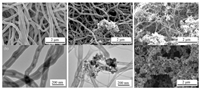
|
Download:
|
| Fig. 1. (a, b) SEM and TEM images of the PDVB nanofiber; (c, d) SEM and TEM images of the PDVB composite synthesized at 0.01 wt% of the Fe3O4 NP; (e, f) SEM images of the composites synthesized at 0.05 wt% and 0.2 wt% of the Fe3O4 NP. DVB and BFEE were fixed at 2 wt% and 0.05 wt%. | |
Growth of the fine nanofibers with polymerization time was monitored to reveal the morphological evolution. At the early stage (30 s), the nanoparticles are coalesced into irregular dendrites (Fig. 2a). The Fe3O4 NPs are encapsulated within PDVB matrix (Fig. 2b). Some short nanofibers of PDVB start to grow from the aggregates surface. The aggregates are stable in good solvents such as THF, implying they are highly crosslinked. With increasing polymerization time (1 min), some short fine nanofibers are clearly distinguished from the aggregates (Fig. 2c). While the nanofibers become longer ~1 μm, more thin nanofibers are grown from the aggregates (Fig. 2d). With prolonged polymerization (5 min), number of the fine PDVB nanofibers is greatly increased (Fig. 2e). A network is formed while the nanofiners become longer and interwoven. After polymerization for 10 min, longer thin nanofibers become major and interwoven a compact network (Fig. 2f). The dendrites are connected with the network. According to the morphological evolution with polymerization, we conjectured a scenery growth of the composites. The cationic polymerization occurs preferentially inside the liquid BFEE droplets forming cationic living polymer chains. When the oleic acid capped Fe3O4 NPs meet the droplets, some cationic living polymer chains are terminated by the carboxyl group at the NPs surface. The process leads an inelastic collision, and the Fe3O4 NPs are engulfed forming the NPs/PDVB composite nanoparticles. The nanoparticles are further coalesced into aggregates and dendrites. The BFEE nanoscale droplets are formed within the PDVB matrix by polymerization induced phase separation. Fine PDVB nanofibers are therefore grown from the aggregates surface in the tip-growth mode.

|
Download:
|
| Fig. 2. SEM and TEM images of the composites after varied polymerization time: (a, b) 30 s; (c, d) 1 min; (e, f) SEM images of the two composites after 5 min and 10 min polymerization. DVB, BFEE and the oleic acid capped Fe3O4 NP were fixed at 2 wt%, 0.05 wt% and 0.05 wt%. | |
The Fe3O4 NPs are encapsulated within the PDVB network, rendering magnetic responsive behavior. Magnetization of the composites is determined by the content of Fe3O4. In the presence of 0.05% of Fe3O4 NP, the amount of thin nanofibersreaches maximum while exhibiting sufficient magnetic performance. The thin PDVB nanofiber remains the similar hydrophobic as the thick PDVB nanofiber (Fig. S2-1 in Supporting information). The PDVB composite nanofibers keep the brown color when emerging in acidic water, while water keeps colorless (Fig. S2-2 in Supporting information). No leakage of Fe3O4 occurs, ensuring the composite nanofibers are stable in water treatment. In comparison, the Fe3O4 NPs absorbed PDVB nanofibers become white from the original brown, and water becomes yellow (Fig. S2-3 in Supporting information). The absorbed Fe3O4 NPs are completely dissolved in acidic water. Similar with our previous report, the current thin PDVB composite nanofibers can effectively absorb the representative chemicals forming the gels (Fig. 3a). As shown in Table S1 (Supporting information), the thin PDVB nanofiber displays higher absorption capability for all the chemicals. In comparison, the PDVB dendrite exhibits weaker absorption capability. The interconnected network of the PDVB nanofibers and toluene can be distinguished when visualized at different wavelength under confocal laser scanning microscope. At the 488 nm fluorescence channel, the network of PDVB nanofibers displays red (Fig. 3b1). At the 559 nm fluorescence channel, the background displays green corresponding to the toluene phase at the interstitial voids within the network (Fig. 3b2). The high gelation capability of the nanofibers is promising to collect chemical spills from water. Dyed toluene was used as the model chemical spill on water (Fig. 3c1). Upon adding the PDVB nanofibers, toluene was captured forming a gel (Figs. 3c1–2). The collection was too fast within 30 s to monitor the absorption process. The gel was completely removed with a magnet (~0.8 T) (Fig. 3c3). Neither toluene nor gel fragment was residual in water. The saturated adsorption capacity for toluene was 24.0 mL/g. The magnetic PDVB nanofiber was easily regenerated by squeezing and then evaporation of the chemicals at high temperature. When the temperature is close to the boiling point of the organic solvent, most of the solvent can be removed. The saturated adsorption capacity was slightly decreased to 22.9 mL/g after 15 cycles (Fig. 3d). The magnetic collection capability of the composite nanofibers keeps stable even after stored in air for one year.

|
Download:
|
| Fig. 3. (a) Gelation for some representative chemicals with the PDVB composite nanofiber: (1) water, (2) cyclohexane, (3) carbon tetrachloride, (4) dimethylformamide, (5) toluene, (6) ethanol; (b) CLSM images of the toluene captured gel: (1) the PDVB nanofiber (red) at the 488 nm fluorescence channel, (2) toluene (green) at the 559 nm fluorescence channel; (c) (1) toluene (top) on water (bottom), oil soluble dye Sudan Ⅲ was added in toluene for easier observation, (2) capturing toluene after addition of the PDVB composite nanofiber, (3) collection of the gel with a magnet; (d) collection capability of the PDVB composite nanofiber with cycles. | |
In summary, we prepared magnetic responsive PDVB nanofiber in the presence of oleic acid capped Fe3O4 NP during the precipitated cationic polymerization. The Fe3O4 NPs are encapsulated with crosslinked PDVB and immobilized within the nanofibers. The magnetic performance of nanofiber composites is determined by the content of Fe3O4, which is well kept in air for one year. The nanofiber composites exhibit strong gelation capability for all representative chemicals. The performance is promising for magnetic collection of chemical spills. The magnetic nanofiber composites can be further modified to introduce ligands and used to collect metal ions with a magnet.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.002.
| [1] |
Z.Y. Wu, C. Li, H.W. Liang, et al., Sci. Rep. 4 (2014) 4079-4079. DOI:10.1038/srep04079 |
| [2] |
H.C. Bi, Z.Y. Yin, X.H. Cao, et al., Adv. Mater. 25 (2013) 5916-5921. DOI:10.1002/adma.201302435 |
| [3] |
A. Abbas, A.M. Al-Amer, T. Laoui, et al., Sep. Purif. Technol. 157 (2016) 141-161. DOI:10.1016/j.seppur.2015.11.039 |
| [4] |
K. Yoon, B.S. Hsiao, B. Chu, J. Mater. Chem. 18 (2008) 5326-5334. DOI:10.1039/b804128h |
| [5] |
Y.S. Zhou, D.Z. Yang, X.M. Chen, et al., Biomacromolecules 9 (2008) 349-354. DOI:10.1021/bm7009015 |
| [6] |
C.J. Luo, M. Nangrejo, M. Edirisinghe, Polymer 51 (2010) 1654-1662. DOI:10.1016/j.polymer.2010.01.031 |
| [7] |
D. Lv, M.M. Zhu, Z.C. Jiang, et al., Macromol. Mater. Eng. 303 (2018) 1800336. DOI:10.1002/mame.201800336 |
| [8] |
A. Greiner, J.H. Wendorff, Angew. Chem. Int. Ed. 46 (2007) 5670-5703. DOI:10.1002/anie.200604646 |
| [9] |
W. Ni, F.X. Liang, J.G. Liu, et al., Chem. Commun. 47 (2011) 4727-4729. DOI:10.1039/c1cc10900f |
| [10] |
D.M. Lv, W. Ni, F.X. Liang, et al., Chin. J. Polym. Sci. 33 (2015) 1344-1350. DOI:10.1007/s10118-015-1683-2 |
| [11] |
S.Q. Cui, X.Y. Ji, F.X. Liang, Z.Z. Yang, Chin. Chem. Lett. 26 (2015) 942-945. DOI:10.1016/j.cclet.2015.04.034 |
| [12] |
G.Z. Li, W.C. Peng, X.Y. Li, et al., Appl. Surf. Sci. 254 (2008) 4970-4979. DOI:10.1016/j.apsusc.2008.01.167 |
 2020, Vol. 31
2020, Vol. 31 
