Developing an alternative energy coupling approach for catalysis, complementary to conventional heat-based chemical transformations that require the consumption of fossil fuels, is receiving tremendous attention due to the increasing energy and environmental problems. Harvesting sunlight for catalysis is a promising solution to coupling solar energy into chemical transformations [1, 2], which can be accomplished through either photocatalysis or photothermal-driven catalysis toward organic synthesis [3, 4]. While photocatalysis has been extensively explored, photothermal-driven catalysis is still at the early stage. In order to fully harvest sunlight and convert it to heat, the material should have the ability of absorbing photons in a broad spectrum, particularly in the visible and near-infrared (NIR) regions. Semiconducting π-conjugated microporous polymers (CMPs) are very promising in converting incident photons into localized heat [5], suggesting that they are potentially useful in photothermal-driven catalysis. We specifically would like to employ such a photothermal agent to drive catalytic reactions which usually require an external heat source or/and high pressure.
Among various chemical transformations, alcohol plays an important role in the production of fine and specialty chemicals. The selective oxidation of alcohols to carbonyl compounds such as aldehydes, carboxylic acids and esters is considered as a fundamentally important industrial process [6-8]. In particular, benzaldehyde has been known as one of the most important products, widely used in medicine, dye, fragrance and resin industry [9]. Conventionally, the oxidations of alcohols are carried out using stoichiometric oxidants such as chromates or permanganates, involving toxicity-related environmental problems. With the aim to overcome these drawbacks, oxidation of benzyl alcohol using molecular oxygen (O2) has been proposed for benzaldehyde production as a eco-friendly and cost-effective process [4, 10, 11]. In the past decades, great efforts have been devoted to achieving efficient alcohol oxidation using heterogeneous noble metal catalysts such as Au [12, 13], Pt [14, 15] and Pd [16, 17], whose catalytic performance can be enhanced by forming bimetallic nanoparticles owing to their unique properties such as intermetallic charge transfer and geometrical interactions [18-21]. For instance, Hutchings and co-workers [22] reported that benzyl alcohol oxidation using bimetallic AuPd can reach a conversion rate of 90% under 120 ℃ as the synergistic effect between Au and Pd nanoparticles could endow them with intense electronic and geometrical interactions to promote the catalytic reaction [23-26]. However, the high performance of benzyl alcohol oxidation by AuPd typically requires relatively high reaction temperature by an external heat source, significantly increasing the energy input of this catalytic system. Ideally, a photothermal-driven catalytic system can achieve efficient benzyl alcohol oxidation without the need of external heat source.
π-Conjugated microporous polymers (CMPs) have demonstrated their unique chemical nature in catalysis and other applications [27, 28]. Aza-fused CMP (aza-CMP) is a new type of two-dimensional (2D) polymer with an extended π-conjugated network [29]. Theoretical calculations indicated that aza-CMP possessed a low bandgap of 1.07 eV, implying that it is a low-bandgap semiconductor with broad spectral absorption. Meanwhile, the inherent porous structure of aza-CMP can be used as a new class of efficient support for heterogeneous catalysts. For example, Co(OH)2 was deposited on aza-CMP to boost the photocatalytic oxygen evolution performance of aza-CMP [30]. Nevertheless, the implementation of aza-CMP in catalysis was mainly focused on the utilization of its large surface area and porous structure [31], while its unique optical-related properties such as photothermal effect have yet to be fully explored for catalytic applications. Currently, many other types of photothermal materials have been extensively studied; however, there are some unavoidable obstacles in their applications. For example, organic compounds, such as cyanine dyes and porphyrin, may suffer from severe photobleaching during their application [32]; various gold nanostructures have strong NIR absorption, but the high cost and limited reserve of Au restrict their practical applications while Au nanorods have low photostability due to the "melting effect" [33]. In contrast, aza-CMP exhibited outstanding stability, and can be prepared simply and conveniently [28, 30].
Herein, aza-CMP supported AuPd nanocatalysts were prepared through a simple sol-immobilization method for light-driven oxidation of benzyl alcohol. Inherited from aza-CMP, the aza-CMP/AuxPdy exhibited large specific surface area and unique porous structure. More importantly, aza-CMP can absorb incident light and convert it to heat through a photothermal effect, promoting catalytic reactions. Consequently, the aza-CMP/AuxPdy composite showed an outstanding performance in benzyl alcohol oxidation even without direct heating. This approach opens the possibility of substituting light-driven reactions for conventional thermal-based chemical production.
Aza-CMP nanosheets were synthesized according to previous study [30] by the condensation reaction between 1, 2, 4, 5-benzenetetramine tetrahydrochloride and hexaketocyclohexane octahydrate in N-methyl-2-pyrrolidone (Fig. S1 in Supporting information). As shown in scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images (Figs. 1a and b), the as-obtained aza-CMP exhibited a sheet-like structure, which would facilitate the following deposition of AuPd nanoparticles. Moreover, it was found that the prepared aza-CMP sheets were agglomerated due to their strong interlayer interaction (Fig. 1b).

|
Download:
|
| Fig. 1. (a) SEM and (b) TEM images of aza-CMP. (c) SEM and (d) TEM images of aza-CMP/Au1Pd2. | |
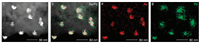
Subsequently, the aza-CMP sheets were deposited with AuPd nanoparticles through a co-reduction method. Upon the co-reduction deposition, the sheet-like structure of aza-CMP was preserved with additional nanoparticles on the surface (Figs. 1c and d). This indicates that the unique physical properties of aza-CMP can be sustained after the AuPd deposition. The successful deposition of AuPd on the prepared sample was further confirmed by energy-dispersive spectroscopy (EDS) mapping profiles on scanning transmission electron microscope (STEM). As shown in Fig. 2, Au and Pd elements were homogeneously distributed on aza-CMP nanosheets.
|
Download:
|
| Fig. 2. (a) STEM image of aza-CMP/Au1Pd2 and (b–d) its corresponding EDS mapping profiles for AuPd (b), Au (c) and Pd (d). | |
The crystalline and phase structures of samples were further studied by X-ray diffraction (XRD) characterization. As shown in Fig. S2 (Supporting information), aza-CMP is an amorphous material with irregularly stacked nanosheets according to the broad peak at 25°-30°. After the deposition of AuPd nanoparticles, distinct XRD diffraction peaks located between the standard patterns for face-centered cubic (fcc) Au (JCPDS 04-0784) and Pd (JCPDS No. 46-1043) can be observed, suggesting the formation of AuPd alloy on the surface of aza-CMP nanosheets. The surface chemical composition of aza-CMP was also determined using X-ray photoelectron spectroscopy (XPS) as shown in Fig. S3 (Supporting information). Apparently, the obtained aza-CMP material contained C and N elements, showing both C—N and C=N bonding structure. Moreover, Fourier-transform infrared spectroscopy (FT-IR) further demonstrated the bonding of C and N in the polymer (Fig. S4 in Supporting information). Obvious absorption peaks attributed to C=C and C=N were observed to confirm the condensation of amino group with carbonyl group. These functional groups on the surface of aza-CMP can act as trapping sites for the growth of metal nanoparticles, limiting their sizes. To have a full picture on the aza-CMP structure, 13C cross-polarization magic angle spinning (CP-MAS) solid-state nuclear magnetic resonance (NMR) spectroscopy was employed to characterize the sample. As shown in Fig. S5 (Supporting information), three different carbon ring structures were identified for the aza-CMPs, in which the peaks at 141, 134, and 114 ppm corresponded to the carbon atoms of the phenyl edges connected to aza units, the carbon atoms of the triphenylene cores on vertices, and the unsubstituted carbon atoms of the phenyl edges, respectively. The XRD, XPS and NMR characterization results are in good agreement with previous reports [34], further confirming the successful preparation of aza-CMP/AuxPdy composite. It is worth noting that the pore size of aza-CMP is below 2 nm [30, 34], while the size of AuPd nanoparticles in this work is about 35 nm. Thus the AuxPdy nanoparticles in aza-CMP/AuxPdy composite should be loaded on aza-CMP instead of embedded in aza-CMP.
As indicated in previous reports [22], bimetallic AuxPdy nanoparticles are effective catalysts for oxidation reaction of benzyl alcohol with superior catalytic performance to monometallic nanoparticles. To evaluate the benzyl alcohol oxidation performance of our samples, the obtained aza-CMP/Au, aza-CMP/Pd and aza-CMP/AuxPdy were used to catalyze the oxidation of benzyl alcohol under 20–60 ℃ heating. As displayed in Figs. 3a–c, aza-CMP/Pd showed relatively low catalytic activity for benzyl alcohol oxidation. By incorporating Au into the catalyst, the performance of benzyl alcohol oxidation was dramatically promoted with the increase of Au:Pd ratio up to 1:2 in aza-CMP/AuxPdy. It is believed that this promotion is enabled by the synergistic effect between Au and Pd. Pd atoms served as the sites for O2 activation, while the addition of Au atoms can isolate the Pd sites to tune O2 adsorption [35]. However, further increasing the ratio of Au to Pd led to a decrease in benzyl alcohol oxidation performance as the overloading of Au atoms reduced the number of Pd sites for O2 activation. Moreover, it should be noted that the optimized aza-CMP/AuPd2 composite exhibited a high selectivity toward the production of benzaldehyde which reached 90% at 50 ℃ heating. It worth noting that the conversion of catalytic benzyl alcohol oxidation under 50 ℃ heating is 98.2% with selectivity of 90.3%; however, the selectivity under 60 ℃ is reduced to 64.8% at similar conversion. This indicates that the reaction temperature of 50 ℃ is an optimized reaction temperature.

|
Download:
|
| Fig. 3. Catalytic performance for benzyl alcohol oxidation by different samples under (a) 50 ℃, (b) 40 ℃ and (c) 30 ℃ heating in dark condition. (d) Durability test of aza-CMP/Au1Pd2 for four cycles under 50 ℃ heating in dark condition. | |
Upon recognizing the catalytic activity of samples, we are in a position to investigate the catalytic performance of benzyl alcohol oxidation by the optimized aza-CMP/AuxPdy (i.e., aza-CMP/Au1Pd2) using simulated light irradiation instead of external heat source. Considering the actual sunlight irradiation intensity reaching the earth surface [36], a low-power light source (light intensity = 50 mW/cm2) was used during the test. As shown in Fig. 4a, the catalytic performance of benzyl alcohol oxidation by aza-CMP/Au1Pd2 under 50 mW/cm2 light irradiation is comparable to that under 50 ℃ heating and significantly higher than that at 20–40 ℃. This manifests that the aza-CMP/Au1Pd2 catalyst possesses remarkable performance in catalytic benzyl alcohol oxidation without an external heat source, demonstrating its great potential in replacing the thermal-driven catalytic process. It is believed that this performance can be attributed to the photothermal effect brought by the aza-CMP near AuPd catalysts.

|
Download:
|
| Fig. 4. (a) Catalytic performance for benzyl alcohol oxidation by aza-CMP/Au1Pd2 at different condition. (b) Photothermal curves of water with and without aza-CMP (at a concentration of 3 mg/mL) with the evolution of time under 50 mW/cm2 light irradiation. (c) Catalytic performance for benzyl alcohol oxidation by aza-CMP/Au1Pd2 under light irradiation with different light intensity at room temperature. (d) Catalytic performance for styrene hydrogenation by aza-CMP/Au1Pd2 under light irradiation with different light intensity at room temperature. | |
To look into the energy source for such an external heat source-free benzyl alcohol oxidation by aza-CMP/Au1Pd2, we collected UV–vis-NIR diffuse reflectance spectrum of aza-CMP nanosheets (Fig. S6 in Supporting information). The spectrum indicates that aza-CMP can absorb light in a wide spectral range, especially in the visible and infrared light region, demonstrating the capability of aza-CMP in effectively harvesting sunlight. Although the harvested photons cannot directly participate in benzyl alcohol oxidation reaction, it can induce a localized heat spot for AuPd nanoparticles to facilitate the reaction. To further prove the photothermal effect brought by aza-CMP on the catalytic system, we recorded the photothermal conversion by dispersing aza-CMP in water under light illumination as shown in Fig. 4b and Fig. S7 (Supporting information). In the absence of aza-CMP, a negligible temperature change can be observed in the solution system. In contrast, when the aza-CMP was introduced into this system, the temperature of water can be increased up to 9 ℃. This temperature increase is attributed to the broadband light absorption by the aza-CMP which can in turn convert the absorbed light into heat. It worth mentioning that the measured temperature increase for water should be a final outcome resulting from the heat transfer from the aza-CMP surface to the surrounding water. For this reason, the local temperature near the AuPd nanoparticle surface should be significantly higher than that measured in solution shown by Fig. 4b. As a result, the catalytic performance in Fig. 4a, driven by the photothermal effect, reached the level comparable to 50 ℃ external heating. This result confirms that aza-CMP exhibits an excellent photothermal effect which can play its dominant role in achieving light-driven benzyl alcohol oxidation using aza-CMP/Au1Pd2 composite.
The information gleaned above has well demonstrated that the incident light played an important role in promoting the benzyl alcohol oxidation reaction by aza-CMP/Au1Pd2. We further performed systematic investigations to gain a comprehensive understanding on the effect of incident light on catalytic benzyl alcohol oxidation. Fig. 4c shows the catalytic performance of aza-CMP/Au1Pd2 in benzyl alcohol oxidation using the light source with different light intensity (25–450 mW/cm2). At low light intensity (25 mW/cm2), the conversion of benzyl alcohol is limited by insufficient energy source. Meanwhile, the aza-CMP/Au1Pd2 sample displayed the gradually reduced benzyl alcohol conversion rate while the selectivity for benzaldehyde production was slightly promoted by increasing light intensity from 50 mW/cm2 to 450 mW/cm2. This dependence of conversion rate and selectivity on light intensity may result from light-induced plasmonic hot electrons. Under a high-intensity irradiation of light, hot electrons can be generated on the surface of bimetallic nanoparticles via localized surface plasmon resonance [37], which may transfer to aza-CMP. The decrease in electron density on AuPd surface is not preferable for O2 molecular activation [26], reducing the oxidation ability. As a result, the aza-CMP/Au1Pd2 showed the suppressed conversion rate under 450 mW/cm2 light irradiation, but the selectivity toward benzaldehyde production which did not require a high density of active oxygen species was slightly improved.
To further evaluate the effect of hot electrons on chemical reactions catalyzed by aza-CMP/Au1Pd2, we also performed catalytic styrene hydrogenation using aza-CMP/Au1Pd2 under different light intensities. As proven in a previous report, a low electron density on Pd-based catalysts is beneficial for hydrogenation reactions [38]. As such, a higher conversion rate observed for styrene hydrogenation can serve as an indicator for the lower electron density on metal nanocatalysts in aza-CMP/Au1Pd2. As displayed in Fig. 4d, the performance of aza-CMP/Au1Pd2 in the hydrogenation of styrene to ethylbenzene was promoted by increasing light irradiation intensity. This feature suggests the reduction of electron density on the Au1Pd2 under intense light irradiation.
In summary, we have successfully synthesized aza-CMP supported bimetallic AuPd nanoparticles for external heat source-free benzyl alcohol oxidation. The aza-CMP/Au1Pd2 showed excellent performance in benzyl alcohol oxidation toward benzaldehyde using simulated light irradiation. This light-driven performance of aza-CMP/Au1Pd2 is attributed to the photothermal effect brought by aza-CMP nanosheets, which could increase the surrounding temperature of reaction system. Such a photothermal-driven approach can be extended to other reaction systems such as hydrogenation. This work demonstrates an alternative route to catalyze reactions by replacing external heating with light illumination.
AcknowledgmentsThis work was supported by National Key R&D Program of China (Nos. 2017YFA0207301, 2017YFA0207302), the National Natural Science Foundation of China (NSFC, Nos. 21725102, 21601173, U1832156, 21881240040, 21573212), CAS Key Research Program of Frontier Sciences (No. QYZDB-SSW-SLH018), CAS Interdisciplinary Innovation Team, and Chinese Universities Scientific Fund (No. WK2310000067). J. Low was funded by Chinese Academy of Sciences President's International Fellowship Initiative (No. 2019PC0114). We thank the support from USTC Center for Micro- and Nanoscale Research and Fabrication.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.022.
| [1] |
P. Zhang, J. Zhang, J. Gong, Chem. Soc. Rev. 43 (2014) 4395-4422. DOI:10.1039/C3CS60438A |
| [2] |
G. Chen, R. Gao, Y. Zhao, et al., Adv. Mater. 30 (2018) 1704663. DOI:10.1002/adma.201704663 |
| [3] |
S. Linic, U. Aslam, C. Boerigter, M. Morabito, Nat. Mater. 14 (2015) 567-576. DOI:10.1038/nmat4281 |
| [4] |
N. Zhang, X. Li, H. Ye, et al., J. Am. Chem. Soc. 138 (2016) 8928-8935. DOI:10.1021/jacs.6b04629 |
| [5] |
J. Tan, J. Wan, J. Guo, C. Wang, Chem. Commun. 51 (2015) 17394-17397. DOI:10.1039/C5CC05478H |
| [6] |
D.I. Enache, J.K. Edwards, P. Landon, et al., Science 311 (2006) 362-365. DOI:10.1126/science.1120560 |
| [7] |
X. Jiang, J. Zhang, S. Ma, J. Am. Chem. Soc. 138 (2016) 8344-8347. DOI:10.1021/jacs.6b03948 |
| [8] |
H. Su, K.X. Zhang, B. Zhang, et al., J. Am. Chem. Soc. 139 (2017) 811-818. DOI:10.1021/jacs.6b10710 |
| [9] |
D. Han, T. Xu, J. Su, X. Xu, Y. Ding, ChemCatChem 2 (2010) 383-386. DOI:10.1002/cctc.201000001 |
| [10] |
X. Wang, C. Wang, Y. Liu, J. Xiao, Green Chem. 18 (2016) 4605-4610. DOI:10.1039/C6GC01272H |
| [11] |
Y. Yan, X. Jia, Y. Yang, Catal. Today 259 (2016) 292-302. DOI:10.1016/j.cattod.2015.07.021 |
| [12] |
D. Tsukamoto, Y. Shiraishi, Y. Sugano, et al., J. Am. Chem. Soc. 134 (2012) 6309-6315. DOI:10.1021/ja2120647 |
| [13] |
G. Zhang, R. Wang, G. Li, Chin. Chem. Lett. 29 (2018) 687-693. DOI:10.1016/j.cclet.2018.01.043 |
| [14] |
A. Frassoldati, C. Pinel, M. Besson, Catal. Today 173 (2011) 81-88. DOI:10.1016/j.cattod.2011.02.058 |
| [15] |
C.W. Zhang, L.B. Xu, J.F. Chen, Chin. Chem. Lett. 27 (2016) 832-836. DOI:10.1016/j.cclet.2016.02.025 |
| [16] |
P. O'Brien, D. Lopez-Tejedor, R. Benavente, J.M. Palomo, ChemCatChem 10 (2018) 4992-4999. DOI:10.1002/cctc.201801294 |
| [17] |
S.L. Yu, G. Xuan, Acta Chim. Sinica 61 (2003) 635-640. |
| [18] |
L. Delannoy, S. Giorgio, J.G. Mattei, et al., ChemCatChem 5 (2013) 2707-2716. DOI:10.1002/cctc.201200618 |
| [19] |
M. Chen, D. Kumar, C.W. Yi, D.W. Goodman, Science 310 (2005) 291-293. DOI:10.1126/science.1115800 |
| [20] |
T.A.G. Silva, R. Landers, L.M. Rossi, Catal. Sci. Technol 3 (2013) 2993. DOI:10.1039/c3cy00261f |
| [21] |
T. Balcha, J.R. Strobl, C. Fowler, P. Dash, R.W.J. Scott, ACS Catal. 1 (2011) 425-436. DOI:10.1021/cs200040a |
| [22] |
J. Pritchard, L. Kesavan, M. Piccinini, et al., Langmuir 26 (2010) 16568-16577. DOI:10.1021/la101597q |
| [23] |
A. Savara, C.E. Chan-Thaw, J.E. Sutton, et al., ChemCatChem 9 (2017) 253-257. DOI:10.1002/cctc.201601295 |
| [24] |
S. Guadix-Montero, H. Alshammari, R. Dalebout, et al., Appl. Catal. A 546 (2017) 58-66. DOI:10.1016/j.apcata.2017.07.045 |
| [25] |
W. Ye, S. Chen, M. Ye, et al., Nano Energy 39 (2017) 532-538. DOI:10.1016/j.nanoen.2017.07.025 |
| [26] |
D. Liu, M. Xie, C. Wang, et al., Nano Res. 9 (2016) 1590-1599. DOI:10.1007/s12274-016-1053-6 |
| [27] |
Z.J. Wang, S. Ghasimi, K. Landfester, K.A.I. Zhang, Chem. Mater. 27 (2015) 1921-1924. DOI:10.1021/acs.chemmater.5b00516 |
| [28] |
Y. Xu, S. Jin, H. Xu, A. Nagai, D. Jiang, Chem. Soc. Rev. 42 (2013) 8012-8031. DOI:10.1039/c3cs60160a |
| [29] |
Z.D. Yang, W. Wu, C.Z. Xiao, J. Mater. Chem. C 2 (2014) 2902-2907. DOI:10.1039/C3TC32363C |
| [30] |
L. Wang, Y. Wan, Y. Ding, et al., Nanoscale 9 (2017) 4090-4096. DOI:10.1039/C7NR00534B |
| [31] |
Y. Liao, J. Weber, B.M. Mills, Z. Ren, C.F.J. Faul, Macromolecules 49 (2016) 6322-6333. DOI:10.1021/acs.macromol.6b00901 |
| [32] |
Y. Liu, P. Bhattarai, Z. Dai, X. Chen, Chem. Soc. Rev. 48 (2019) 2053-2108. DOI:10.1039/C8CS00618K |
| [33] |
Z. Zha, X. Yue, Q. Ren, Z. Dai, Adv. Mater. 25 (2013) 777-782. DOI:10.1002/adma.201202211 |
| [34] |
Y. Kou, Y. Xu, Z. Guo, D. Jiang, Angew. Chem. Int. Ed. Engl. 50 (2011) 8753-8757. DOI:10.1002/anie.201103493 |
| [35] |
C. Hu, X. Xia, J. Jin, et al., ChemNanoMat 4 (2018) 467-471. DOI:10.1002/cnma.201800014 |
| [36] |
Q. Lu, J.W. Zhou, Build. Sci. (2012) 22-26. |
| [37] |
R. Long, K. Mao, M. Gong, et al., Angew. Chem. Int. Ed. 53 (2014) 3205-3209. DOI:10.1002/anie.201309660 |
| [38] |
R. Long, Z. Rao, K. Mao, et al., Angew. Chem. Int. Ed. 54 (2015) 2425-2430. DOI:10.1002/anie.201407785 |
 2020, Vol. 31
2020, Vol. 31 
