b Key Laboratory of Hunan Province for Water Environment and Agriculture Product Safety, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Interleukin 2 (IL-2) and IL-2 receptor (IL-2R) play important roles in regulating the magnitude as well as duration of the T-cell immune responses activated by antigens [1]. CD25 is the α-chain of IL-2R that mainly induced and expressed on activated T-cell surface after lymphocyte activation [2]. CD25 can be released from the membrane of activated cells as a smaller soluble form (sCD25) [3]. sCD25 is commonly regarded as one of the most important laboratory parameters that allow monitoring of the immune activation process in vivo. Serum levels of sCD25 are linked with many diseases, such as malignancies, autoimmune diseases and infections [4, 5]. Additionally, sCD25, CD28 and CD38 expression in peripheral blood lymphocytes are used to predict acute rejection after liver transplantation [6]. So, the precise and sensitive detection of sCD25 in serum is of great clinical importance.
Signal amplification is the key for the sensitive detection of trace levels of target analytes in complex samples [7-14]. For electrochemical sensors and biosensors, different redox materials have been studied for signal amplification [15-17]. Among these materials, Prussian blue (PB) is one of the early studied peroxidase-like materials that have high catalytic activity towards H2O2 with low cost. As a result, PB has been widely studied for the preparation of electrochemical biosensors [18-22].
Utilizing the peroxidase-like properties of PB, we synthesized hollow mesoporous Prussian blue nanoparticles (HMPB) and applied the nanoparticles as signal tag for electrochemical detection of sCD25. HMPB was synthesized by acid etching solid PB nanoparticles, which has larger surface area and higher catalytic activity towards H2O2 than solid PB nanoparticles. To facilitate modification of antibodies onto HMPB, gold nanoparticles were deposited onto the HMPB nanoparticle surface (Au@HMPB NPs). The immunosensor was carried out based on traditional sandwich structure, with sCD25 in the sample captured and enriched by antibody modified magnetic Fe3O4 nanospheres. The electrochemical responses of the immunosensors to H2O2 are proportional to the concentration of sCD25 detected.
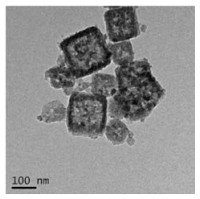
Scheme 1 shows the schematic representation for the synthesis of functionalized HMPB and for the enrichment of sCD25 from sample. The detailed experimental procedures are shown in Supporting information. HMPB was synthesized by etching solid PB nanoparticles with HCl. Initially, PB nanoparticles were synthesized from K3[Fe(CN)6] using polyvinyl pyrrolidone (PVP) as protective agents. To facilitate the surface functionalization of HMPB, gold nanoparticles were deposited onto the HMPB surface for the following adsorption of antibodies. Fig. 1 shows the TEM images of Au@HMPB NPs, which indicates the nanoparticles have cubic shape and large volume hollow cavity with size around 100nm. In addition, the small dot observed at the HMPB surface can be ascribed to gold nanoparticles.

|
Download:
|
| Scheme 1. Schematic representation for the preparation of antibody functionalized magnetic nanospheres, for the preparation of the immuno-probe based on Au@HMPB NPs and for the enrichment of sCD25 from sample. | |
|
Download:
|
| Fig. 1. TEM images of the synthesized Au@HMPB NPs. | |
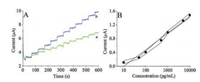
The peroxidase activity of HMPB was then studied. Our previous study has indicated HMPB has higher catalytic activity towards H2O2 than solid PB due to larger surface area of HMPB resulted in increased amount of catalytic sites [23]. For the amperometric measurement, from Fig. 2A, it can be seen at potential of −0.1 V, the HMPB modified electrode has higher response current than solid PB modified electrode towards successive addition of H2O2. This result further proved the high peroxidase activity of HMPB.
|
Download:
|
| Fig. 2. (A) Amperometric responses of the solid PB (curve a) and HMPB (curve b) modified electrode towards successive addition of 1 mmol/L H2O2. Detection potential: −0.1 V. (B) Calibration plot of the immunosensor to different concentrations of sCD25. The response current of the electrodes at −0.1 V to 1 mmol/L H2O2 was collected to draw the calibration plot. The error bars are the standard deviation of measurement results. | |
Detection anti-sCD25 antibodies were directly adsorbed onto the Au@HMPB to transform the nanoparticles into immuno-probe. For the detection of sCD25, capture antibody functionalized magnetic nanospheres were utilized to bind, enrich and isolate sCD25 from samples. After the immobilization of sCD25 linked magnetic nanospheres onto electrode, Au@HMPB can be captured onto electrode through antigen-antibody reaction. The responses of the electrodes to 1 mmol/L of H2O2 are increased with the increasing of the concentration of sCD25 detected (Fig. 2B). A linear relationship between the current response and logarithm of sCD25 concentration in the range from 10 pg/mL to 10 ng/mL is obtained with limit of detection calculated to be 3 pg/mL. The limit of detection is much lower compared to ELISA method (2.15 ng/mL) [2].
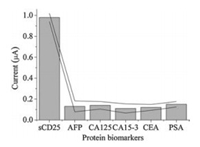
Other analytical performances of the immunosensor, such as selectivity and reproducibility were also studied. To test the selectivity of the immunosensor, the responses of the sensor to several cancer biomarkers including alpha-fetoprotein (AFP), cancer antigen (CA125), cancer antigen 15-3 (CA15-3), carcinoembryonic antigen (CEA) and prostate-specific antigen (PSA) were studied. From Fig. 3, it can be seen that compared to 1 ng/mL of sCD25, the responses of the assay to 10 ng/mL of the above protein biomarkers are negligible, indicating good selectivity of the assay. For reproducibility study, a batch of five parallel immunosensors was prepared under the same experimental conditions to test 0.1 ng/mL and 1 ng/mL of sCD25, respectively. The relative standard deviation of the assay results was 4.1% and 5.2%, respectively, indicating good reproducibility of the immunosensor results.
|
Download:
|
| Fig. 3. Responses of the immunosensor to 1 ng/mL of sCD25 and 10 ng/mL of other protein biomarkers. | |
Based on the above good performance of the assay, a spiked serum sample was performed for detection of sCD25 in human serum samples. A series of concentrations of sCD25 were added into the serum samples and then the samples were analyzed by the assay. Compare the concentrations of sCD25 detected with the amount of sCD25 added, recoveries in the range from 96.2% to 98.3% are obtained (Table 1), proving potential of the assay for clinical applications. The recoveries are all below 100% is probably due to the serum sample matrix effect.
|
|
Table 1 Recovery test of sCD25 in human serum samples (n=3). |
In summary, we studied the performance of Au@HMPB as electrochemical probe for immunosensor utilizing the peroxidase like activity of HMPB. The larger specific surface area of HMPB compared to solid PB resulted in increased catalytic sites, and then enhanced catalytic current towards H2O2 reduction. The surface modification of HMPB with gold nanoparticles facilitated the modification of HMPB with biomolecules. The application of the probe for sCD25 detection resulted in wide linear range and low detection limit. This probe may find wide application for different bioassays.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 21575165).
Appendix A. Supplementary dataSupplementary materialrelatedtothisarticlecanbefound, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2019.05.051.
| [1] |
K.A. Smith, Annu. Rev. Cell Biol. 5 (1989) 397-425. DOI:10.1146/annurev.cb.05.110189.002145 |
| [2] |
Z. Huang, J. Fang, J. Gu, Y. Yan, J. Zhou, Veterinary Immunol. Immunopathol. 140 (2011) 102-109. DOI:10.1016/j.vetimm.2010.11.021 |
| [3] |
L.A. Rubin, F. Galli, W.C. Greene, D.L. Nelson, G. Jay, Cytokine 2 (1990) 330-336. DOI:10.1016/1043-4666(90)90062-X |
| [4] |
S.H. Hsiao, M.S. Lee, H.Y. Lin, et al., Acta Otolaryngol. 129 (2009) 1519-1523. DOI:10.3109/00016480902849427 |
| [5] |
M. Kami, T. Matsumura, Y. Tanaka, et al., Leuk. Lymphoma 38 (2000) 533-540. DOI:10.3109/10428190009059272 |
| [6] |
E. Boleslawski, S. BenOthman, S. Grabar, et al., Clin. Transplant. 22 (2008) 494-501. DOI:10.1111/j.1399-0012.2008.00815.x |
| [7] |
C. Shen, S. Liu, X. Li, D. Zhao, M. Yang, Microchim. Acta 185 (2018) 547. DOI:10.1007/s00604-018-3086-x |
| [8] |
G. Wang, H. Wang, S. Cao, et al., Microchim. Acta 186 (2019) 96. DOI:10.1007/s00604-018-3223-6 |
| [9] |
X. Pei, B. Zhang, J. Tang, et al., Anal. Chim. Acta 758 (2013) 1-18. DOI:10.1016/j.aca.2012.10.060 |
| [10] |
K. Zhang, K. Zeng, C. Shen, S. Tian, M. Yang, Microchim. Acta 185 (2018) 225. DOI:10.1007/s00604-018-2754-1 |
| [11] |
L. Zhao, L. Teng, J. Zhang, H. Li, Chin. Chem. Lett. 30 (2019) 1035-1037. |
| [12] |
Y. Chai, X. Li, M. Yang, Microchim. Acta 186 (2019) 316. DOI:10.1007/s00604-019-3412-y |
| [13] |
Y. Si, Z. Sun, N. Zhang, et al., Anal. Chem. 86 (2014) 10406-10414. DOI:10.1021/ac5028885 |
| [14] |
Y. Si, N. Zhang, Z. Sun, et al., Analyst 139 (2014) 5466-5471. DOI:10.1039/C4AN01074D |
| [15] |
W. Xiang, G. Wang, S. Cao, et al., Microchim. Acta 185 (2018) 335. DOI:10.1007/s00604-018-2867-6 |
| [16] |
X. Li, C. Shen, M. Yang, A. Rasooly, Anal. Chem. 90 (2018) 4764-4769. DOI:10.1021/acs.analchem.8b00023 |
| [17] |
D. Tang, B. Su, J. Tang, J. Ren, G. Chen, Anal. Chem. 82 (2010) 1527-1534. DOI:10.1021/ac902768f |
| [18] |
Y. Zhang, B. Huang, F. Yu, et al., Microchim. Acta 185 (2018) 86. DOI:10.1007/s00604-017-2631-3 |
| [19] |
Y. Lin, L. Hu, L. Yin, L. Guo, Sensor. Actuat. B-Chem. 210 (2015) 513-518. DOI:10.1016/j.snb.2014.12.107 |
| [20] |
R. Li, D. Guo, J. Ye, M. Zhang, Analyst 140 (2015) 3746-3752. DOI:10.1039/C4AN02352H |
| [21] |
W. Zhang, D. Ma, J. Du, Talanta 120 (2014) 362-367. DOI:10.1016/j.talanta.2013.12.028 |
| [22] |
R. Ojani, P. Hamidi, J.B. Raoof, Chin. Chem. Lett. 27 (2016) 481-486. DOI:10.1016/j.cclet.2015.12.030 |
| [23] |
K. Zeng, M. Yang, Y.N. Liu, A. Rasooly, Anal. Methods 10 (2018) 3951-3957. DOI:10.1039/C8AY01456F |
 2020, Vol. 31
2020, Vol. 31 

