b Key Laboratory for Bio-Nanotechnology and Molecular Engineering of Hunan Province, College of Biology, Hunan University, Changsha 410082, China
Photodynamic therapy (PDT), which utilizes photosensitizers generated singlet oxygen (1O2) upon the irradiation with designated wavelengths to induce cancer cell death, has increasing attracted much attention due to its many advantages such as fewer side effects, non-invasive nature, fast healing process and negligible drug resistance [1-4]. However, the therapeutic efficacy of PDT is limited by deep-located tumor cells because of the tissue penetration depth of excitation light [5]. Moreover, the therapeutic efficacy of PDT treatment is also compromised by rapid oxygen depletion and tumor hypoxia microenvironment [6, 7]. In view of these drawbacks, synergistic therapy is usually regarded as a promising approach to enhance the therapeutic efficiency [8-12]. Among various therapies, photothermal therapy (PTT) is the most ideal candidate in clinical cancer treatment [13, 14], which employs photothermal agents to convert near-infrared (NIR) light energy into hyperthermia and then kill cancer cells [15-17]. PTT has many merits, such as minimal invasiveness, low cost and fewer side effects [18-20]. Furthermore, an appropriated level of hyperthermia could increase blood flow of the tumor and thereby improve oxygenation state of the tumor [21-23]. More importantly, NIR light has high tissue penetration ability, so PTT can be used for deep-seated tumor cells [24-26]. Therefore, the development of multimodal synergistic therapy, such as combination of PDT and PTT, will produce much higher anticancer efficacy and harbor the collective merits of respective individual therapies.
Recently, it is really common for the integration of photosensitizers and photothermal adsorbing agents into a nanoassembly to construct the synergistic therapy systems. For example, Han's group reported a combination of photosensitizers-conjugated hyaluronic acid and polydopamine nanoparticles for PDT/PTT dual-modal therapy [27]. Yang and co-workers designed Ce6-decorated black phosphorus nanosheets for PTT/PDT synergistic cancer treatment [28]. Xiang et al. developed Cu2-xS nanoclusters functionalized with Ce6-conjugated branched polyethylenimine for synergistic PTT/PDT therapy [29]. However, synthesis procedures of these nanoparticles are time-consuming, laborious and complicated. Moreover, different components may lead to inestimable mutual interference. Therefore, it is urgent to develop a multifunctional nanoplatform, which can act as photothermal agents and drug delivery vehicles for PDT/PTT cancer synergistic therapy.
Herein, we prepared a Ce6-loaded zinc oxide-polydopamine core-shell nanotherapeutic agent (ZnO@Ce6-PDA) for PTT/PDT synergistic therapy. The Ce6 is the second-generation photosensitizer approved by the Food and Drug Administration (FDA) [30] and has been widely used in PDT treatment on account of the excellent NIR light absorption and high 1O2 generation capability [31, 32]. As shown in Fig. 1, an interlayer of photosensitizer Ce6 was first adsorbed on the surface of ZnO via electrostatic interaction. Subsequently, an outer layer of polydopamine (PDA) was deposited on the surface of ZnO by in situ self-polymerization under alkaline conditions. The obtained core-shell structured ZnO@Ce6-PDA could enter into cells without the help of transfection reagents [33]. The ZnO@Ce6-PDA exhibited outstanding 1O2 generation ability in the presence of 660 nm laser. The ZnO@Ce6-PDA also showed good photothermal conversion efficiency under irradiation with 780 nm. Therefore, it could be considered as a promising nanotherapeutic agent for the synergetic PDT/PTT therapy.

|
Download:
|
| Fig. 1. Schematic illustration of the formation of ZnO@Ce6-PDA and its application in photodynamic/photothermal synergistic therapy of cancer cells. | |
The size and morphology of nanoparticles before and after modification with Ce6 and PDA were characterized. Dynamic light scattering (DLS) measurements demonstrated that the hydrodynamic size of ZnO and ZnO@Ce6 were 46.51 ± 1.44 and 47.75 ± 0.05 nm, respectively (Fig. 2A). The zeta potential was changed from positive (+18.9 ± 1.32 mV) to negative charge (-14.53 ± 0.90 mV) after modifying Ce6 onto the surface of ZnO, indicating the variation of the surface property (Fig. 2B). Compared with the absorption of ZnO, the ZnO@Ce6 exhibited new absorption peaks at 410 and 635 nm which are the characteristic peak of Ce6, suggesting the successful synthesis of ZnO@Ce6 (Fig. 2C).

|
Download:
|
| Fig. 2. (A) DLS measurements of different samples. Inset: Magnified image of a single ZnO@Ce6-PDA. (B) Zeta potentials of different samples. (C) UV-vis absorption spectra of different samples. (D) The fluorescence spectra of different samples. | |
To tune the thickness of the PDA shell, various concentrations of dopaminesolution were mixed with ZnO in Tris buffer. The core-shell structure of ZnO@PDA was observed by transmission electron microscopy (TEM) (Fig. S1 in Supporting information). TEM images showed that ZnO@PDA possessed different physical size and the thickness of the PDA shell (Table S1 in Supporting information). Besides, the coating PDA on the surface of ZnO resulted in a slight red-shift of the absorption peak from 363 nm to 372 nm (Fig. S2 in Supporting information). The prepared ZnO@PDA (3 mg/mL) fell with the size range (50-100 nm) that was better for cellular uptake by mammalian cells [34]. Thus, the ZnO@PDA (3 mg/mL) was applied to the following experiments. The average size of ZnO was 36.91 ± 1.21 nm (Fig. S3A in Supporting information). Typical TEM image showed that the average size of the prepared ZnO@Ce6-PDA was 51.92 ± 1.96 nm and the thickness of the PDA shell was 5.92 ± 0.83 nm (Fig. S3B in Supporting information). Additionally, the hydrodynamic size of ZnO@Ce6-PDA was 76.35 ± 3.06 nm and magnified image of a single ZnO@Ce6-PDA showed obviously core-shell structure (Fig. 2A and inset). Meanwhile, energy-dispersive X-ray spectroscopy (EDS) assay displayed the elements of N in ZnO@Ce6-PDA, implying the successful coating of PDA (Fig. S4 in Supporting information). Compared with ZnO@Ce6, the zeta potential of ZnO@Ce6-PDA was slightly changed to -11.46 ± 0.32 mV, which might be ascribed to the amino groups on the surface of PDA (Fig. 2B). The absorbance intensity of the ZnO@Ce6-PDA was much higher than that of ZnO@Ce6, ascribing to the additiveeffect of the absorbance of PDA from 350 nm to 800 nm (Fig. 2C). The fluorescence intensity at 668 nm of the ZnO@Ce6-PDA was lower than that the same amount of free Ce6, indicating a quenching ability of PDA layer (Fig. 2D). In addition, fourier transform infrared spectra (FT-IR) spectra of ZnO@Ce6-PDA exhibited the absorption band at 3460 cm-1 (stretching vibration of N—H) and 1615 cm-1(bending vibration of C=C), confirming the successful formation of PDA on the ZnO surface (Fig. S5 in Supporting information). To quantify the PDA coating layer and loaded Ce6, thermal gravimetric analyses (TGA) of various nanoparticles were performed. The coating amount of PDA and Ce6 were calculated to be around 8.9 wt% and 6.3 wt%, respectively (Fig. S6 in Supporting information). The time-dependent profile of the normalized hydrodynamic size of the ZnO@Ce6-PDA was presented in Fig. S7A (Supporting information). The size of the ZnO@Ce6-PDA after 24 h had no obvious variation, exhibiting good stability in H2O, PBS or RPMI 1640. Moreover, the ZnO@Ce6-PDA showed no evident precipitation, demonstrating superior monodispersity in the three media (Fig. S7B in Supporting information). There was also no obvious size change for ZnO@Ce6-PDA, indicating excellent stability of ZnO@Ce6-PDA in urine (Fig. S8 in Supporting information).
To investigate the photodynamic property of ZnO@Ce6-PDA, DPBF was employed as an indicator for detecting the generation of 1O2 [35]. When ZnO@Ce6-PDA was irradiated with 660 nm laser (100 mW/cm2) for 30 min, a significant decrease of the DPBF absorption peaks was monitored, indicating the rapid 1O2 generation by ZnO@Ce6-PDA at 660 nm laser (Fig. S9E in Supporting information). In contrast, there were no obvious absorbance changes at 414 nm for DPBF, ZnO or ZnO@PDA, suggesting a negligible production of 1O2 (Figs. S9A-C in Supporting information). Nevertheless, a sharp decline of DPBF absorption at 414 nm for free Ce6 was observed, proving that the 1O2 generation was mainly attributed to the existence of Ce6 molecules (Fig. S9D in Supporting information). Furthermore, Fig. S9F (Supporting information) showed the dynamic process of 1O2 generation by various nanoparticles. Additionally, SOSG was used as an indicator to quantify the 1O2generated by Ce6 and ZnO@Ce6-PDA [36]. The fluorescence intensities of SOSG increased for Ce6 and ZnO@Ce6-PDA in the presence of 660 nm laser (Fig. S10 in Supporting information), implying the successful generation of 1O2. These results adequately confirmed that the ZnO@Ce6-PDA could be regarded as an effective PDT agent.
The photothermal performance of ZnO@Ce6-PDA was then tested. The temperature of the ZnO@PDA and ZnO@Ce6-PDA increased rapidly to 40.5 ℃ after irradiation with 780 nm laser (2.9 W/cm2) for 10 min. In contrast, the temperature of PBS, ZnO, ZnO@Ce6 and Ce6 showed slight changes under the same conditions (Fig. S11A in Supporting information). In addition, the photothermal performance of ZnO@Ce6-PDA was dependent on laser power intensity. The more laser power intensity could generate the higher temperature (Fig. S11B in Supporting information). The temperature of solution increased with the increasing concentrations of ZnO@Ce6-PDA (Fig. S11C in Supporting information). Besides, after continuous 780 nm laser irradiation for four cycles, the process of temperature changes did not show obvious difference, showing a good photothermal stability of ZnO@Ce6-PDA (Fig. S11D in Supporting information). The photothermal performance of various concentrations of ZnO@Ce6-PDA was also monitored in real time by IR thermal imaging (Fig. S11E in Supporting information). To obtain the photothermal conversion efficiency (η), the time-dependent temperature evolutions of the solution were monitored under irradiation (2.9 W/cm2), then naturally cooling to room temperature. The absorbance of different concentrations of ZnO@Ce6-PDA at 780 nm was displayed in Figs. S12A and B (Supporting information). The η was calculated according to the previously reported literature [37]. The obtained η value was about 31.7% for ZnO@Ce6-PDA (Figs. S12C and D in Supporting information). Therefore, ZnO@Ce6-PDA presented an excellent photothermal conversion ability.
To study the cellular uptake and localization of ZnO@Ce6-PDA, HeLa cells were incubated with ZnO@Ce6-PDA. LysoTracker blue was used to stain lysosomes of cells. The cellular uptake and localization of ZnO@Ce6-PDA were observed by confocal laser scanning microscopy (CLSM). As shown in Fig. S13A (Supporting information), with the increasing of incubation time, the fluorescence signals from ZnO@Ce6-PDA (red) were gradually increased, demonstrating great cellular uptake of the ZnO@Ce6-PDA. A well overlapped imaging was monitored between the fluorescence signals from LysoTracker and ZnO@Ce6-PDA (Fig. S13B in Supporting information), suggesting that the majority of ZnO@Ce6-PDA co-localized with the lysosomes. This result proved that the ZnO@Ce6-PDA could enter into cells by endocytosis pathway.
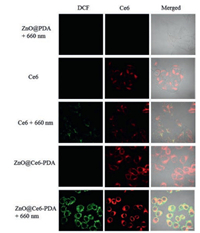
To evaluate the PDT effect, we used the DCFH-DA as an indicator for intracellular 1O2. DCFH-DA can react with the 1O2 and form 2′, 7′-dichlorofluorescein (DCF), which generated green fluorescence at 488 nm laser excitation [38]. As seen in Fig. 3, no green and red fluorescence were detected when HeLa cells were incubated with ZnO@PDA and irradiated for 10 min with 660 nm laser (100 mW/cm2). When HeLa cells were treated with free Ce6 and ZnO@Ce6-PDA, respectively, a strong red fluorescence could be seen inside cells in the absence of 660 nm laser. And the fluorescence signal of ZnO@Ce6-PDA was stronger than that of free Ce6, indicating the higher internalization of ZnO@Ce6-PDA. Obvious green fluorescence was noted in cells treated with free Ce6 and ZnO@Ce6-PDA in the presence of 660 nm laser, suggesting the generation of 1O2 in living cells. The excellent 1O2 production ability of ZnO@Ce6-PDA in cancer cells showed the potential application for the PDT.
|
Download:
|
| Fig. 3. CLSM images of intracellular 1O2 generation of HeLa cells treated with ZnO@PDA, Ce6 and ZnO@Ce6-PDA and irradiated with or without a 660 nm laser (100 mW/cm2). Scale bar = 20 μm. | |
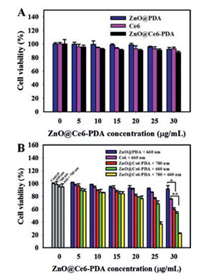
The cytotoxicity of nanoparticles was evaluated by MTT assay. Three types of nanoparticles, including ZnO@PDA, Ce6 and ZnO@Ce6-PDA, were respectively incubated with HeLa cells. The viability of cells remained more than 90% at the concentration of 30 μg/mL after 24 h of incubation, implying an excellent biocompatibility of the three nanoparticles (Fig. 4A). Additionally, we explored the PTT effects of 780 nm NIR laser on HeLa cells. The viability of the cells remained over 94% after irradiating by the 780 nm laser at the power densities of 0.77, 1.84 and 2.9 W/cm2, respectively. However, when the power density was 3.94 W/cm2, an obvious phototoxicity was noted (Fig. S14 in Supporting information). Thus, we selected the power density of 2.9 W/cm2 to perform the PTT in the following experiments.
|
Download:
|
| Fig. 4. (A) Viability of HeLa cells treated with different concentrations of ZnO@PDA, Ce6 and ZnO@Ce6-PDA. (B) Viability of HeLa cells treated with ZnO@PDA, free Ce6 and ZnO@Ce6-PDA under the irradiation with 660 nm (100 mW/cm2) and/or 780 nm (2.9 W/cm2), respectively. (*P < 0.05, **P < 0.01). | |
Furthermore, the synergistic PDT/PTT was investigated by incubating HeLa cells with various concentrations of ZnO@Ce6-PDA under the irradiation with 660 nm and/or 780 nm laser. Viabilities of HeLa cells after treatment were demonstrated in Fig. 4B. For PTT alone, cells were treated with various concentrations of ZnO@Ce6-PDA. The PTT effect of ZnO@Ce6-PDA under 780 nm laser (2.9 W/cm2, 10 min) was dose-dependent. Cell viability was 60.1% at the concentration of 30 μg/mL. For PDT alone, cells treated with ZnO@Ce6-PDA were irradiated with 660 nm laser for 10 min (100 mW/cm2). It was found that cell viability decreased to 53.8% at the concentration of 30 μg/mL, which was much lower than that of cells incubated with the equivalent concentration of ZnO@PDA (91.5%) and free Ce6 (75.9%). The result showed that the ZnO@Ce6-PDA showed good PDT effect. For the synergistic PDT/PTT, HeLa cells treated with various concentrations of ZnO@Ce6-PDA were irradiated with 660 and 780 nm laser. Cell viability was declined to about 22.1%, indicating the excellent synergistic therapeutic effect.
In summary, we successfully developed a simple and versatile nanotherapeutic agent for the combined PDT/PTT therapy of cancers. As expected, the ZnO@Ce6-PDA was equipped with high 1O2 generation and excellent photothermal conversion efficiency. Moreover, the ZnO@Ce6-PDA indicated outstanding phototoxicity and excellent biocompatibility. Furthermore, in vitro experimental results revealed that the ZnO@Ce6-PDA effectively killed cancer cells by the combination of PDT/PTT synergistic therapy, which were more effective than any single treatment modality. The ZnO@PDA nanoparticles can be further constructed to load drugs or biomolecules to design promising multifunctional nanoassemblies for future cancer therapy and clinical application.
AcknowledgmentsThis work was supported in part by the Key Project of Natural Science Foundation of China (Nos. 21775036, 21675046, 21735002, 21521063 and 21874035), the Key Point Research and Invention Program of Hunan Province (No. 2017DK2011), the Research Foundation of Education Bureau of Hunan Province (No. 18B027) and the Hunan Provincial Natural Science Foundation (No. 2018JJ2033).
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.05.004.
| [1] |
D. He, L. Hai, X. He, et al., Adv. Funct. Mater. 27 (2017) 1704089. DOI:10.1002/adfm.201704089 |
| [2] |
N. Li, T. Li, C. Hu, et al., ACS Appl. Mater. Interfaces 8 (2016) 15013-15023. DOI:10.1021/acsami.5b02037 |
| [3] |
H. Chen, J. Tian, W. He, et al., J. Am. Chem. Soc. 137 (2015) 1539-1547. DOI:10.1021/ja511420n |
| [4] |
J.F. Lovell, T.W.B. Liu, J. Chen, et al., Chem. Rev. 110 (2010) 2839-2857. DOI:10.1021/cr900236h |
| [5] |
W. Fan, P. Huang, X. Chen, Chem. Soc. Rev. 45 (2016) 6488-6519. DOI:10.1039/C6CS00616G |
| [6] |
Z. Zhou, J. Song, L. Nie, et al., Chem. Soc. Rev. 45 (2016) 6597-6626. DOI:10.1039/C6CS00271D |
| [7] |
Z. Huang, H. Xu, A.D. Meyers, et al., Technol. Cancer Res. Treat 7 (2008) 309-320. DOI:10.1177/153303460800700405 |
| [8] |
S. Wang, X. Zhao, J. Qian, et al., ACS Appl. Mater. Interfaces 8 (2016) 24368-24384. DOI:10.1021/acsami.6b05907 |
| [9] |
B. Jang, J.Y. Park, C.H. Tung, et al., ACS Nano 5 (2011) 1086-1094. DOI:10.1021/nn102722z |
| [10] |
J. Lin, S. Wang, P. Huang, et al., ACS Nano 7 (2013) 5320-5329. DOI:10.1021/nn4011686 |
| [11] |
L. Gao, J. Fei, J. Zhao, et al., ACS Nano 6 (2012) 8030-8040. DOI:10.1021/nn302634m |
| [12] |
S. Yang, L. Zhou, Y. Su, et al., Chin. Chem. Lett. 30 (2019) 187-191. DOI:10.1016/j.cclet.2018.02.015 |
| [13] |
C. Liang, S. Diao, C. Wang, et al., Adv. Mater. 26 (2014) 5646-5652. DOI:10.1002/adma.201401825 |
| [14] |
Q. Chen, C. Liang, C. Wang, et al., Adv. Mater. 27 (2015) 903-910. DOI:10.1002/adma.201404308 |
| [15] |
Y. Liu, G. Shu, X. Li, et al., Adv. Funct. Mater. 28 (2018) 1802026. DOI:10.1002/adfm.201802026 |
| [16] |
X. Ding, J. Liu, J. Li, et al., Chem. Sci. 7 (2016) 6695-6700. DOI:10.1039/C6SC01320A |
| [17] |
Y. Liu, K. Ai, J. Liu, et al., Adv. Mater. 25 (2013) 1353-1359. DOI:10.1002/adma.201204683 |
| [18] |
H. Liu, D. Chen, L. Li, et al., Angew. Chem. Int. Ed. 50 (2011) 891-895. DOI:10.1002/anie.201002820 |
| [19] |
J. Wang, Y. Dong, Y. Li, et al., Adv. Funct. Mater. 28 (2018) 1707360. DOI:10.1002/adfm.201707360 |
| [20] |
Q. Li, Y. Sun, L. Ren, et al., ACS Appl. Mater. Interfaces 10 (2018) 29314-29324. DOI:10.1021/acsami.8b09330 |
| [21] |
Y. Wang, Y. Song, G. Zhu, et al., Chin. Chem. Lett. 29 (2018) 1685-1688. DOI:10.1016/j.cclet.2017.12.004 |
| [22] |
W. Cheng, J. Nie, N. Gao, et al., Adv. Funct. Mater. 27 (2017) 1704135. DOI:10.1002/adfm.201704135 |
| [23] |
L. Ao, C. Wu, K. Liu, et al., ACS Appl. Mater. Interfaces 10 (2018) 12544-12552. DOI:10.1021/acsami.8b02973 |
| [24] |
B. Liu, C. Li, B. Xing, et al., J. Mater. Chem. B 4 (2016) 4884-4894. DOI:10.1039/C6TB00799F |
| [25] |
D. Zhang, M. Wu, Y. Zeng, et al., ACS Appl. Mater. Interfaces 7 (2015) 8176-8187. DOI:10.1021/acsami.5b01027 |
| [26] |
W. Fan, B. Yung, P. Huang, et al., Chem. Rev. 117 (2017) 13566-13638. DOI:10.1021/acs.chemrev.7b00258 |
| [27] |
J. Han, W. Park, S.J. Park, et al., ACS Appl. Mater. Interfaces 8 (2016) 7739-7747. DOI:10.1021/acsami.6b01664 |
| [28] |
X. Yang, D. Wang, Y. Shi, et al., ACS Appl. Mater. Interfaces 10 (2018) 12431-12440. DOI:10.1021/acsami.8b00276 |
| [29] |
H. Xiang, F. Xue, T. Yi, et al., ACS Appl. Mater. Interfaces 10 (2018) 16344-16351. DOI:10.1021/acsami.8b04779 |
| [30] |
M.G. Adimoolam, M.R. Nalam, M.V. Sunkara, et al., J. Mater. Chem. B 5 (2017) 9189-9196. DOI:10.1039/C7TB02599H |
| [31] |
L. Feng, L. Cheng, Z. Dong, et al., ACS Nano 11 (2017) 927-937. DOI:10.1021/acsnano.6b07525 |
| [32] |
J. Li, K. Wei, S. Zuo, et al., Adv. Funct. Mater. 27 (2017) 1702108. DOI:10.1002/adfm.201702108 |
| [33] |
M. Sun, L. Xu, P. Fu, et al., Adv. Funct. Mater. 26 (2016) 7352-7358. DOI:10.1002/adfm.201601942 |
| [34] |
W. Jiang, B.Y.S. Kim, J.T. Rutka, et al., Nat. Nanotech. 3 (2008) 145-150. DOI:10.1038/nnano.2008.30 |
| [35] |
Y. Cai, P. Liang, Q. Tang, et al., ACS Nano 11 (2017) 1054-1063. DOI:10.1021/acsnano.6b07927 |
| [36] |
Q. Chen, C. Li, X. Yang, et al., J. Mater. Chem. B 5 (2017) 7529-7537. DOI:10.1039/C7TB01590A |
| [37] |
Q. Tian, R. Zou, Q. Liu, et al., ACS Nano 5 (2011) 9761-9771. DOI:10.1021/nn203293t |
| [38] |
N.A. Daghastanli, R. Itri, M.S. Baptista, Photochem. Photobiol. 84 (2008) 1238-1243. DOI:10.1111/j.1751-1097.2008.00345.x |
 2020, Vol. 31
2020, Vol. 31 

