Doxcycline hyclate (DC) is a second generation teracycline antibiotics that widely used in human healthcare and animal feeds. DC has broad-spectrum antibacterial activities against both Gram-negative and Gram-positive bacteria and shows good treatment effect on several bacteritic infections [1-4]. However, the long-period abuse of antibiotics caused serious public concerns. With the extensive use of DC for medicinal applications, several side effects have been found [5-7]. The monitoring of antibiotics in human serum is meaningful due to the abuse of antibiotics leads to serious health problems and bacterial resistance [8-10]. How to develop new methods for DC analysis in biological samples still remains challenging. Various methods have been established for the determination of DC, such as high-performance liquid chromatography [11], capillary electrophoresis [12], enzyme linked immunosorbent assay (ELISA) [13], fluorescence spectrophotometric method [14]. However, most of these methods mentioned above are either time-consuming or demand complicated instrumentations [15].
Molecularly imprinting technology (MIT) has been widely explored in electrochemical sensors because the introduction of MIT can enhance selectivity of the sensor [16, 17]. Molecularly imprinted polymers (MIPs) are known as composite materials that co-polymerized in the presence of target molecules and possess specific binding sites to target molecules [18]. After target molecules removed through chemical elution or physical methods, binding sites left within MIPs are complementary in size, and structure towards target molecules, which can specifically identify target molecules [19, 20]. Comparing with other techniques, electro-polymerization attracted significant interests in electrochemical analysisbecause of ease of preparation and good reproducibility [21, 22]. However, the introduction of MIPs in electrochemical sensors dramatically reduces conductivityof the sensor, which makes electrochemical sensors based on MIPs lack of sensitivity to the target molecules [23]. Efforts had been made to solve this issue. For example, through combination of MIPs with conductive nanomaterials is an efficient solution [24-26].
The unique structural, mechanical and electronic properties of multi-walled carbon nanotubes (MWCNTs) have shown promising applications in various sensing areas [27, 28]. MWCNTs have been used to combine with MIPs for electrode modification since MWCNTs can significantly enhance the conductivity of the sensor [29, 30].
In this study, a novel DC imprinted poly(o-phenylenediamine) (P-o-PD) electrochemical sensor based on MWCNTs modified glassy carbon electrode was reported. o-Phenylenediamine can be easily electro-polymerized onto electrode surface to form imprinted film that provides binding sites for DC molecules. MWCNTs were utilized to enhance the electrochemical response sensitivity. The detection of DC was achieved through suppressing of the sensor current intensity in ferricyanide solution after binding of DC molecules onto electrode surface.
For preparing the sensor, 0.5 mg MWCNTs was dispersed into 1.0 mL of DMF to prepare a homogeneous suspension. Then, 5 μL of the MWCNTs suspension was dropped onto the glassy carbon electrode (GCE) surface and dried under infrared lamp to obtain MWCNTs/GCE.
The obtained MWCNTs/GCE was immersed into 0.1 mol/L HAc/NaAc buffer solution (pH 6.0) containing 10 mmol/L o-phenylenediamine and 5 mmol/L DC. Then cyclic voltammetry (CV) was employed for electrochemical polymerization of P-o-PD in the potential range of 0 V-0.80 V at a scan rate of 50 mV/s for 20 cycles. For removing template molecules, the fabricated MIP sensor was dipped into the mixture of methanol-acetic acid (8:1, v/v) for 15 min. As a contrast, the non-imprinted sensor was prepared exactly the same way but without adding DC.
During the formation of the imprinted film, o-PD was electro-polymerized onto the electrode surface. At the same time, DC, as template molecules were trapped into the MIP matrix by means of reversible interactions, including π-π interaction and hydrogen bonds between MIPs and DC molecules [31]. DC is a tetracyclinederivative that involves several functional groups on its structure, such as phenyl ring, hydroxyl, tertiary amine, carbonyl, etc. Rudimental amino in the polymer can binding oxygen and nitrogen atoms in the DC molecules via hydrogen bond [32]. The shape and size of the imprinted cavities were specific to DC molecules after the removal of the template. The schematic representation of the sensor preparation and detection process is illustrated in Scheme 1.

|
Download:
|
| Scheme 1. The electrochemical polymerization of the MIP film, the elution and detection process of the sensor. | |
As shown in Fig. 1A, an irreversible anodic oxidation peak appeared at potential of 0.4 V in the initial CV scan for the electro-polymerization of o-PD, which is attributed to the oxidation of o-PD. Subsequence decrease of the current peak occurred when the scan cycles increased, indicating the formation of P-o-PD film, which was reported non-conductive [24]. The non-conductive film can significantly hinder the electron transfer when formed on the electrode surface. With the thickness of the film augmented, more and more DC molecules were trapped inside the electro-polymerized imprinted polymer.

|
Download:
|
| Fig. 1. (A) Cyclic voltammograms for electro-polymerization of the molecularly imprinted film. (B) Cyclic voltammograms of bare GCE (a), MWCNTs/GCE (b), MIPs/MWCNTs/GCE without the addition of template (c), MIPs/MWCNTs/GCE with the addition of template (d), and MIPs/ MWCNTs/GCE after the elution of DC molecules (e) in 5.0 mmol/L [Fe(CN)6]3-/4-. (C) EIS of bare GCE (a), MWCNTs/GCE (b), MIPs/MWCNTs/GCE (with template molecule) (c), MIPs/MWCNTs/GCE (removing template molecule) (d), MIPs/MWCNTs/GCE (rebinding DC) (e) in 5.0 mmol/L [Fe(CN)6]3-/4- solution with 0.1 mol/L KCl. | |
The sensor modification process was also characterized in detail in [Fe(CN)6]3-/4- solution by CV. As shown in Fig. 1B, after adding MWCNTs onto GCE surface, the current response of the MWCNTs/GCE (curve b) was higher than that of bare GCE (curve a), indicating the MWCNTs on the GCE surface increased the conductibility and surface area of the electrode. MIPs/MWCNTs/GCE without the addition of template (curve c) and MIPs/MWCNTs/GCE with the template (curve d) almost have no redox peaks, indicating that the P-o-PD film is a compact non-conductive membrane which blocked the electron transfer. However, after removal of the template, MIPs/MWCNTs/GCE (curve e) shows clear redox peak, indicating that the cavities were exist in the MIPs membrane after elution that allows [Fe(CN)6]3-/4- to reach the electrode surface.
Electrochemical impedance spectroscopy (EIS) is another efficient method for monitoring the changes of the electrode surface. Fig. 1C shows the impedance curves of the electrode under various conditions. It can be seen compared to bare electrode (curve a), MWCNTs/GCE (curve b) displays smaller semicircle in the Nyquist plot, indicating that the MWCNTs significantly accelerated the electron transfer. After the electro-polymerization of MIPs film onto electrode surface (curve c), the impedance of the electrode increased, suggesting the MIPs film blocked the electron transfer. After removing the template, the impedance decreased (curve d), proving that the template was successfully removed and the left cavities facilitated the electron access to the electrode surface. After rebinding of DC, there is an increase of the impedance again (curve e), indicating that the DC molecules binding to cavities on the MIPs thus prevented the electron transfer.
The thickness of the MIP film affects the sensitivity of the sensor, which can be influenced by varying the electro-polymerization time. While thicker MIP film can provide abundant imprinted sites to enhance the sensitivity of the sensor, however, the DC molecules probably cannot be removed completely from the prepared sensors if too thick MIP film covered on the electrode surface. Fig. 2A shows the effect of the number of CV scan cycles on the sensitivity of the sensor. When the scan cycles increased from 0 to 20, molecularly imprinted film was formed on the electrode surface, so that more and more specific recognition sites were immobilized. When scan cycles increased more than 20, the thickness of non-conductive film improved thus hamper electron transfer, so the current decreased quickly. The sensitivity of the sensor increased with the increase of scan cycles and reach maximum value at 20 cycles. Thus, 20 cycles was chosen as the optimum number of CV scan cycles for electro-polymerization.

|
Download:
|
| Fig. 2. Effect of electro-polymerization time (A), different molar ratios of template molecule to o-PD monomer (B), supporting electrolyte pH (C) and different incubation time (D) on the sensitivity of the sensor. | |
The amounts of template molecules trapped within MIPs during polymerization depended on the molar ratio of template molecule to o-PD monomer. To obtain the optimum molar ratio, different sensors were prepared using 4 different molar ratios (2:1, 1:1, 1:2 and 1:3) with concentration of template molecule fixed at 5 mmol/L. As shown in Fig. 2B, the sensor fabricated at a molar ratio of 1:2 exhibits the highest sensitivity. It can be explained that excess o-PD monomer forms non-conductive membrane without recognition sites when the molar ratio was too low, while excess template molecules cannot interacted with polymer matrix because lack of functional monomers. Therefore, the optimal molar ratio of template molecules to o-PD monomers of 1:2 was chosen for the following experiments.
To study the optimal pH value for the experiment, the pH value in the range from 3.0 to 7.0 was tested. The response of the sensor increased with pH value increased up to 6.0 and then decreased gradually (Fig. 2C). It is because too acidic or alkaline environment can influence the integrity of the imprinted film to lower the sensor sensitivity. So pH 6.0 was chosen as optimal pH value.
For the incubation time of the sensor with DC sample, as shown in Fig. 2D, the current response of the sensor increased as incubation time increased and reached a plateau at 15 min. After that, no further increase of sensitivity was observed, indicating that DC molecules are completely bound to the cavities of the MIP membrane. Hence, the optimal incubation time was set at 15 min.
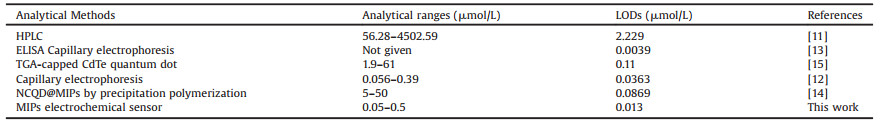
Under optimized experimental conditions, the sensor responses toward different concentrations of DC were investigated. As shown in Fig. 3A, the peak current of the sensor decreased linearly with the DC concentration increased. Fig. 3B shows the linear range from 0.05 μmol/L to 0.5 μmol/L with a limit of detection (LOD) of 1.3 × 10-2 μmol/L that calculated based on S/N = 3. The performance of the sensor for DC detection was compared to literature reports. As shown in Table 1, obviously, this sensor is more sensitive than previously reported methods. The result indicates that the MIPs/MWCNTs/GCE sensor has good potential for DC quantitative detection.

|
Download:
|
| Fig. 3. (A) DPV responses of the sensor to different concentrations of DC (from a to g: blank, 0.05 μmol/L, 0.1 μmol/L, 0.2 μmol/L, 0.3 μmol/L, 0.4 μmol/L, 0.5 μmol/L). (B) Calibration plot of the sensor to different concentrations of DC. (C) The current response of the sensor to 0.2 μmol/L DC and analogues. | |
|
|
Table 1 Comparison of different methods for detection of DC. |
The selectivity of the sensor was evaluated by testing the responses of the sensor toward tetracycline, ofloxacin, amoxicillin and cloxacillin. These compounds have similar chemical structure and property to DC. As shown in Fig. 3C, the sensitivity of the sensor toward DC is much higher than that to the above compounds, indicating this sensor possesses high selectivity to DC. The specificity was probably attribute to imprinted polymer matrix formed certain shaped cavities which just perfectly matched DC molecules, so that other analogues could not bind to MIPs.
The reproducibility of the sensor was estimated by measuring 0.2 μmol/L DC with 5 different electrodes that prepared under the same condition can be found in Fig. S1 (Supporting information). The relative standard deviation (RSD) of the testing results was 2.18%. As shown in Fig. S2 (Supporting information), the repeatability of the sensor was also examined by testing 0.2 μmol/L DC using the same sensor for 6 times. The RSD was 2.9% for the six successive assays. After each assay, the sensor was washed by the methanol-acetic acid (8:1, v/v) for 15 min, and the current peak values of the sensor was recorded for six time, the RSD was 0.6%, which suggested that our method has a good elution efficiency towards DC (Fig. S3 in Supporting information). The stability of sensor was also investigated (Fig. S4 in Supporting information). The sensor lost only 5.4% of sensitivity after stored in refrigerator at 4 ℃ for 3 weeks, exhibiting good long-term stability.
Finally, the sensor was applied to detect DC in human serum samples. DC in human serum was analyzed by the standard addition method. Five different concentrations of DC (0.05 μmol/L, 0.10 μmol/L, 0.15 μmol/L, 0.20 μmol/L) were added into human serum samples for testing. The assay results were summarized in Table S1 (Supporting information). The recovery of DC in human serum was in the range of 95.07%-105.4%. It can be seen that the sensor has the ability for determining DC in complicated real samples.
In summary, a high sensitive and selective electrochemical sensor for detection of DC based on MIPs and MWCNTs was developed. MWCNTs modified electrodeprovides large surface area for molecular imprinting and high conductivity for electron transfer. After the electro-polymerization of MIPs under the optimum conditions, the sensor shows good performance for DC detection. The sensor was successfully applied to determine DC in human serum samples with good recovery results, which revealed promising clinical applications of the sensor.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (No. 21575165).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.026.
| [1] |
E. Buduneli, S. Vardar-Sengül, N. Buduneli, et al., J. Periodont. 78 (2007) 127-134. DOI:10.1902/jop.2007.050451 |
| [2] |
M. Mishra, B. Mishra, Acta Pharm. Sin. B 2 (2012) 518-526. DOI:10.1016/j.apsb.2012.05.001 |
| [3] |
B. Sloan, N. Scheinfeld, Expert Opin. Drug Saf. 7 (2008) 571-577. DOI:10.1517/14740338.7.5.571 |
| [4] |
D. Vargas-Estrada, J. Gracia-Mora, H. Sumano, Res. Vet. Sci. 84 (2008) 477-482. DOI:10.1016/j.rvsc.2007.07.003 |
| [5] |
E. Ayaslioglu, E. Erkek, A. Oba, E. Cebecioglu, Aust. Dent. J. 50 (2005) 273-275. DOI:10.1111/j.1834-7819.2005.tb00373.x |
| [6] |
F. Stickel, H.K. Seitz, J. Gastrointest. Liver Dis. 22 (2013) 189-197. |
| [7] |
S. Thiele-Bruhn, J. Plant Nutr. Soil Sci. 166 (2003) 145-167. DOI:10.1002/jpln.200390023 |
| [8] |
A. Ito, T. Sato, M. Ota, et al., Antimicrob. Agents Chemother. 62 (2018) 1-11. |
| [9] |
H. Ji, H. Sun, X. Qu, Adv. Drug Deliv. Rev. 105 (2016) 176-189. DOI:10.1016/j.addr.2016.04.009 |
| [10] |
E.Y. Klein, T.P. van Boeckel, E.M. Martinez, et al., Proc. Natl. Acad. Sci. U. S. A. 115 (2018) 3463-3470. DOI:10.1073/pnas.1717295115 |
| [11] |
E. Patyra, E. Kowalczyk, K. Kwiatek, Bull. Vet. Inst. Pulawy 56 (2012) 329-333. DOI:10.2478/v10213-012-0058-5 |
| [12] |
G. Islas, J.A. Rodriguez, I. Perez-Silva, J.M. Miranda, I.S. Ibarra, J. Anal. Methods Chem. 2018 (2018) 1-7. |
| [13] |
J. Adrian, F. Ferna'ndez, F. Sa'nchez-Baeza, M.P. Marco, J. Agric. Food Chem. 60 (2012) 3837-3846. DOI:10.1021/jf2053355 |
| [14] |
X. Feng, J. Ashley, T. Zhou, Y. Sun, Microchim. Acta 185 (2018) 500-508. DOI:10.1007/s00604-018-2999-8 |
| [15] |
J. Tashkhourian, G. Absalan, M. Jafari, S. Zare, Spectroc. Acta A 152 (2016) 119-125. DOI:10.1016/j.saa.2015.07.063 |
| [16] |
K. Haupt, K. Mosbach, Chem. Rev. 100 (2000) 2495-2504. DOI:10.1021/cr990099w |
| [17] |
M. Ulbricht, J. Chromatogr. B 804 (2004) 113-125. DOI:10.1016/j.jchromb.2004.02.007 |
| [18] |
P. Wang, X. Fu, J. Li, et al., Chin. Chem. Lett. 22 (2011) 611-614. DOI:10.1016/j.cclet.2010.12.004 |
| [19] |
S. Ansari, Trac-Trends Anal. Chem. 93 (2017) 134-151. DOI:10.1016/j.trac.2017.05.015 |
| [20] |
A.G. Mayes, Trac-Trends Anal. Chem. 16 (1997) 321-332. DOI:10.1016/S0165-9936(97)00037-X |
| [21] |
J. Bai, X. Zhang, Y. Peng, et al., Sensor. Actuat. B-Chem. 238 (2017) 420-426. DOI:10.1016/j.snb.2016.07.035 |
| [22] |
L. Yang, B. Xu, H. Ye, F. Zhao, B. Zeng, Sensor. Actuat. B-Chem. 251 (2017) 601-608. DOI:10.1016/j.snb.2017.04.006 |
| [23] |
Z.Z. Yin, S.W. Cheng, L.B. Xu, et al., Biosens. Bioelectron. 100 (2018) 565-570. DOI:10.1016/j.bios.2017.10.010 |
| [24] |
H. Dai, D. Xiao, H. He, et al., Microchim. Acta 182 (2014) 893-908. |
| [25] |
E. Roy, S. Patra, A. Tiwari, R. Madhuri, P.K. Sharma, Biosens. Bioelectron. 89 (2017) 234-248. DOI:10.1016/j.bios.2016.02.056 |
| [26] |
Y. Yin, L. Yan, Z. Zhang, J. Wang, Talanta 144 (2015) 671-679. DOI:10.1016/j.talanta.2015.06.067 |
| [27] |
R.H. Baughman, A.A. Zakhidov, W.A. de Heer, Science 297 (2002) 787-792. DOI:10.1126/science.1060928 |
| [28] |
R.J. Chen, Y. Zhang, D. Wang, H. Dai, J. Am. Chem. Soc. 123 (2001) 3838-3839. DOI:10.1021/ja010172b |
| [29] |
H. Wang, S. Yao, Y. Liu, S. Wei, J. Su, G. Hu, Biosens. Bioelectron. 87 (2017) 417-421. DOI:10.1016/j.bios.2016.08.092 |
| [30] |
L. Zhang, Y. Wen, Y. Yao, et al., Chin. Chem. Lett. 25 (2014) 517-522. DOI:10.1016/j.cclet.2013.12.020 |
| [31] |
G. Yang, F. Zhao, B. Zeng, Biosens. Bioelectron. 53 (2014) 447-452. DOI:10.1016/j.bios.2013.10.029 |
| [32] |
F. Wang, L. Zhu, J. Zhang, Sensor. Actuat. B-Chem. 192 (2014) 642-647. DOI:10.1016/j.snb.2013.11.037 |
 2020, Vol. 31
2020, Vol. 31