The long-term and increasing application of chemical pesticides caused a series of problems, such as pesticide toxicity residues, environmental pollution and ecological balance damage. Simultaneously, the use cycle of pesticides would be shortened due to the quick renewal and the existence of receptor resistance. Therefore, the research of new pesticides would be based on low toxicity, environmental protection, high efficiency and reduction of resistance [1]. The avermectins are a group of 16-membered ring macrocyclic lactones with broad spectrum of insecticidal, acaricidal and anthelmintic activities, which were isolated from the fermentation broth of Streptomyces avermitilis [2]. Avermectins directly act on the γ-aminobutyric acid (γ-GABA) chloride ion channels, which are unique to insects, arachnids, nematodes and ticks, so they possess extremely low toxicity to mammals [3, 4]. Avermectins have become the main product of the insecticidemarket for cash crops because of remarkable activities, complex structure and intriguing mechanism of action [5, 6].
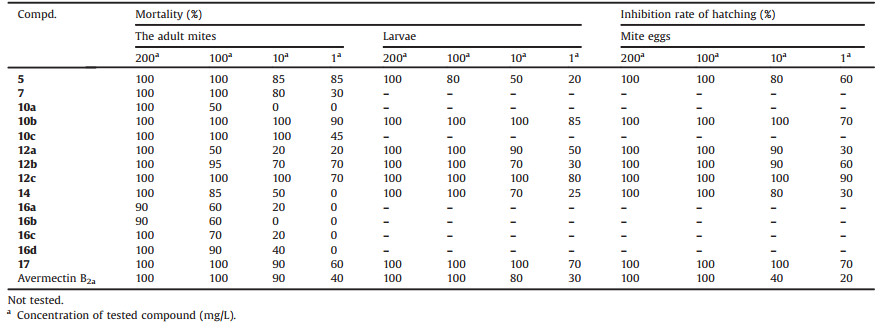
Since the discovery of avermectins, great efforts have been made to obtain new derivatives with higher activity and lower toxicity by modifying the avermectin B1a molecule (Fig. 1, A) and some of avermectin B1a derivatives have been commercial products [7]. For example, ivermectin (Fig. 1, B) not only possesses same remarkable anthelmintic activity as avermectin B1a but also shows better permeability and safety for mammalian [8]. Besides, the insecticidal activity of emamectin benzoate (Fig. 1, C) against beet armyworm is 1500 times that of avermectin B1a [9]. However, rare attention has been concentrated on structural modification of avermectin B2a (Fig. 1, D), which also possesses excellent bioactivities and better safety [10]. Thence, it is feasible to reform the structure of avermectin B2a and develop novel avermectin B2a derivatives.
|
Download:
|
| Fig. 1. The chemical structures of compounds A–F. | |
The glycosidic bond of the oleandrose disaccharide is easily broken and the bare 5- and 23-position hydroxyl groups are easily oxidized, which would lead to the extreme unstability of avermectin B2a molecule. Some studies [11, 12] showed that milbemectin (Fig. 1, E) and moxidectin (Fig. 1, F) without the oleandroose disaccharide exhibited extremely outstanding biological activity and safety, and these derivatives have been commercialized for agricultural use. In addition, due to the wide application of the ester group, hydrazide group, hydrazone group and oxime ether group in a plurality of active drug molecules, so in this paper, fourteen novel avermectin B2a aglycon derivatives in which the above active groups were introduced at 13-, 5- and 23-position after removing the oleandrose disaccharide of avermectin B2a were designed and synthesized. The insecticidal, acaricidal and fungicidal activities of all target compounds were evaluated accordingly. Three dimensional quantitative structure-activity relationship (3D-QSAR) was discussed to understand the relationship between the molecule structure and bioactivity of avermectin B2a agylcon derivatives.
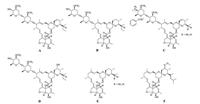
The synthesis of compounds 5 and 7 was shown in Scheme 1. Avermectin B2a aglycon (2) was synthesized according to the reported literature [13] with improvements. During which process, it was found that the yield of compound 2 was highly correlated with the concentration of sulfuric acid in methanol and the amount of solvent. Allyl chloroformate (Alloc-Cl) and N, N, N′, N′-tetramethylethylene diamine (TMEDA) were dropwise added into compound 2 in dichloromethane (CH2Cl2) at low temperature giving compound 3, which reacted with methanesulfonyl chloride (MsCl) in the presence of TMEDA at 0–5 ℃ to give compound 4. The process of the synthesis of compound 4 was carried out in two steps. First, the mesylation at 13-position hydroxyl group was conducted. Second, the mesylate group was easy to leave in the presence of TMEDA to form compound 4. A stable conjugation with four C=C bond was formed which was favor both in kinetics and thermodynamics. In the presence of triethylamine (TEA), 3 was then acylated with ethoxy oxalylchloride in dichloromethane (CH2Cl2) at 0–5 ℃ to afford compound 6. Finally, the 5- and 23-position protecting groups of 4 and 6 were removed with tetrakis(triphenylphosphine)palladium and NaBH4 in mixed solution of isopropyl acetate and ethanol (isopropyl acetate:ethanol = 7:3) to give compounds 5 and 7.
|
Download:
|
| Scheme 1. Synthesis of target compounds 5 and 7. | |
As shown in Scheme S1 (Supporting information), compound 3 was oxidized with Dess-Martin periodinane (DMP) under the protection of N2 to obtain compound 8. Then, the reaction of 8, hydroxylamine hydrochloride and pyridine in ethanol (EtOH) at reflux temperature gave compound 9a, which could convert to 10a using the same method as described for compounds 5 and 7. The compounds 12a-c and 14 were prepared according to the route as shown in Scheme S2 (Supporting information). Compound 2 was oxidized by Dess-Martin periodinane (DMP) at 0 ℃ under the protection of N2 and simultaneously gave compounds 11 and 13, which were identified by 13C NMR spectra. The 5-position carbonyl group is conjugated to a C=C double bond, while the 23-position carbonyl group is isolated. The chemical shift of C5 (δ 192.57) is smaller than that of C23 (δ 203.92). Compounds 11 and 13 were refluxed with methoxyammonium chloride in ethanol at the presence of pyridine to give compounds 12b and 14, respectively.
The synthetic procedure of 16a-d and 17 was shown in Scheme S3 (Supporting information). Compound 8 was reacted with methyl carbazate, pyridine and glacial acetic acid (AcOH), which could activate 13-position carbonyl group, to give compound 15a. Then the protected groups were removed using the same method as described above for compound 5 and compound 16a was obtained. 16a was reduced with sodium cyanoborohydride (NaBH3CN) in etanol (EtOH) to give compound 17.
The bioactivities of target compounds were determined, and the methods used for the insecticidal and acaricidal activities of these compounds were based on published studies [14]. The test method of in vitro fungicidal activities was shown in Supporting information.
The insecticidal activities of all compounds against diamondback moth and aphid were shown in Tables S1 and S2, respectively (Supporting information). From Table S1, all of the target compounds expect for 16b showed 100% mortality against diamondback moth at 200 mg/L. Compounds 10c and 16a exhibited 100% mortality at 100 mg/L, but when the concentration was reduced to 10 mg/L and 1 mg/L, all of compounds revealed low insecticidal activity against diamondback moth. Based on the results in Tables S1 and S2, the insecticidal activity of the target compounds against aphids was not as good as that against diamondback moth. Only 12c (100%, 100 mg/L; 50%, 10 mg/L) showed better insecticidal activity against aphids than avermectin B2a (90%) at 100 mg/L, and possessed the same mortality as avermectin B2a (50%) at 10 mg/L.
The acaricidal activity of target compounds was displayed in Table 1. The results revealed that some compounds exhibited obvious lethal activities against the adult mites, larvae and good inhibition rate of hatching to mite eggs of Tetranychus cinnabarinus at 200 mg/L and 100 mg/L. Notably, compounds 10b, 10c and 12cdemonstrated 100% mortality against adult mites at 10 mg/L, which were superior to avermectin B2a (90%). Besides, the mortality of compounds 5, 10b, 10c, 12b, 12cand 17 was 85%, 90%, 45%, 70%, 70% and 60% at 1 mg/L, respectively, which were better than that of avermectin B2a (40%). For compounds 5, 10b, 12a-c, 14, 17 with excellent activity, their acaricidal activities against larvae and inhibition rate of hatching to mite eggs of Tetranychus cinnabarinus were further investigated and the results were listed in the right of Table 1. It is worth mentioning that the activities of compounds 10b, 12a, 12c, 17 against larvae and mite eggs of Tetranychus cinnabarinus were all greater than avermectin B2a at 10 mg/L and 1 mg/L. In addition, the activity of compound 5 against mite eggs of Tetranychus cinnabarinusreached 70% inhibition rate of hatching at 1 mg/L, which was higher than 20% of avermectin B2a.
|
|
Table 1 Acaricidal activity against the adult mites, larvae and hatching inhibition rate to mite eggs of Tetranychus cinnabarinus. |
Fungicidal activity of title compounds against fourteen plant fungal pathogens in vitro at 50 mg/L were determined, the results were shown in Tables S3 and S4 (Supporting information). The fungicidal activity of compound 10b was the best. Except for the Physalospora piricola, the inhibition rates of 10b against other thirteen fungal pathogens exceeded to that of avermectin B2a and were similar to that of chlorothalonil, in which the inhibition rate against Alternaria solani reached 65.4%. In addition, compounds 5 and 10c all exhibited higher fungicidal activities against various fungal pathogens such as Gibberella zeae in comparison with avermectin B2a, while their fungicidal activities were not as good as that of chlorothalonil.
Based on the data of bioassay, compound 5 had good biological activity, probably because of its unique eight-electron conjugated system and the absence of hydrogen bonding at 13-position. To some extent, the introduction of oxime ether groups at the 13- and 23-position of avermectin B2a aglycon increased its acaricidal and fungicidal activity. The larger the substituent, the better activity it has. The order of mortality or inhibition rate are = NOC2H5 (10c, 12c) > =NOCH3 (10b, 12b) > =NOH (10a, 12a). But the insecticidal activity after introducing an oxime ether group at 5-position (14) was not good as expected, which might result from the destruction of the hydrogen bond structure formed by the 5-position hydroxyl group with the acceptor. None of the compounds 16a-d containing hydrazone groups showed better biological activity, while compound 17 with hydrazine group in molecule showed more excellent activity than the compounds containing hydrazone groups.
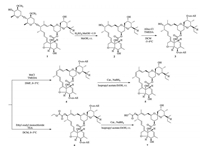
CoMFA was one of the main analytical methods to investigate 3D-QSAR of drug design and the CoMFA model was generated based on bioactivities of drug molecules [15-17]. In this paper, comprehensive CoMFA analyses was performed in a similar method as that in previous study [18]. The results based on computation were summarized in Table S5 (Supporting information). As shown in Table S5, the cross-validation coefficient q2 and the correlation coefficients R2 of CoMFA model are 0.53 and 0.954, respectively, which indicated that the model have an excellent predictive ability. In addition, the contribution of the steric field and the electrostatic field are 67.4% and 32.6%, respectively, which indicated that the steric field has a greater influence on the bioactivity of the compounds. A comparison of experimental and predicted activity against Rhizoctonia cerealis by CoMFA for the target compounds was exhibited in Table S6 (Supporting information) and the linearity of them was described in Fig. 2. The correlation coefficients R2 of CoMFA model was 0.940, which was closing to that in the calculation, demonstrating that the model was fairly accurate and further proving that the model had a good predictive ability.
|
Download:
|
| Fig. 2. Plot of experimental and predicted results of fungicidal activity against Rhizoctonia cerealis for CoMFA model. | |
The CoMFA contour maps were illuminated in Fig. 3. Blue contour in the CoMFA electrostatic field as showed in Fig. 3A meant regions where electropositive groups would increase activities, conversely, the red contour indicated regions where electropositive groups would decrease activities. The green area in the CoMFA steric field as displayed in Fig. 3B denoted regions where bulky groups would heighten activities and the yellow region where smaller groups would heighten activities [19, 20]. It could be seen from Fig. 3 that the introduction of bulky and electronegative groups at 13-position of avermectin B2a aglycon was beneficial to increase the fungicidal activity of the molecule, which was consistent with the experimental results and greatly helpful to the further structural modification of avermectin B2a aglycon.
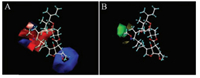
|
Download:
|
| Fig. 3. CoMFA contour maps of electrostatic (A) and steric (B) fields. | |
In summary, fourteen unreported avermectin B2a aglycon derivatives were synthesized and were confirmed by 1H NMR, 13C NMR and HRMS. The bioassays showed that some of the target compounds exhibited excellent acaricidal activities against adult mites (5, 10b, 10c, 12b, 12c, 17) and larvae (10b, 12a, 12c, 17) of Tetranychus cinnabarinus, good inhibition rate of hatching to mite eggs of Tetranychus cinnabarinus (5, 10b, 10c, 12a-c, 17) and high inhibition rate against various fungi (5, 10b, 10c). CoMFA model was successfully constructed based on the activities of target compounds against Rhizoctonia cerealis, which could accurately predict the fungicidal activities of avermectin B2a aglycon derivatives and provided a powerful tool for the synthesis of high-activity compounds.
AcknowledgmentThis work was supported by the National Key R & D Program of China (No. 2018YFD0200100).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.03.054.
| [1] |
G.S. Sun, J.J. Zhang, S.H. Jin, J.J. Zhang, RSC Adv. 8 (2018) 3774-3781. DOI:10.1039/C7RA13258A |
| [2] |
Q.A. Wu, Z.Y. Xu, M. Zheng, et al., Chin. Chem. Lett. 15 (2004) 765-767. |
| [3] |
S.S. Pong, C.C. Wang, L.C. Fritz, J. Neurochem. 34 (2010) 351-358. |
| [4] |
W.L. Shoop, H. Mrozik, M.H. Fisher, Vet. Parasitol. 59 (1995) 139. DOI:10.1016/0304-4017(94)00743-V |
| [5] |
Y.M. Jia, X.M. Liang, X.Q. Fang, et al., Chin. Chem. Lett. 18 (2007) 895-898. DOI:10.1016/j.cclet.2007.06.003 |
| [6] |
P. Sun, Q.F. Zhao, F.T. Yu, et al., J. Am. Chem. Soc. 135 (2013) 1540-1548. DOI:10.1021/ja311339u |
| [7] |
J. Zhang, X. Nan, H.T. Yu, et al., Eur. J. Med. Chem. 121 (2016) 422-432. DOI:10.1016/j.ejmech.2016.05.056 |
| [8] |
W.C. Campbell, Curr. Pharm. Biotechnol. 13 (2012) 853-865. DOI:10.2174/138920112800399095 |
| [9] |
M.H. Fisher, ACS Symp. Ser. 524 (1993) 169. |
| [10] |
H. Mrozik, P. Eskola, M.H. Fisher, et al., J. Med. Chem. 25 (1982) 658-663. DOI:10.1021/jm00348a010 |
| [11] |
R. Prichard, C. Ménez, A. Lespine, Int. J. Parasitol: Drugs Drug Resist. 2 (2012) 134-153. DOI:10.1016/j.ijpddr.2012.04.001 |
| [12] |
A.P. Liu, J.R. Yao, Agrochem. 43 (2004) 196-200. |
| [13] |
H. Mrozik, P. Eskola, B.H. Arison, J. Org. Chem. 47 (1982) 489-492. DOI:10.1021/jo00342a023 |
| [14] |
J.H. Song, Synthesis and Bioactivity Study and SAR of Natural Product Harmaline and Harmine and their Derivatives, Doctoral Dissertation, Nankai University, 2014.
|
| [15] |
E.R. Collantes, W. Tong, W.J. Weish, W.L. Zielinski, Anal. Chem. 68 (1996) 2038-2043. DOI:10.1021/ac951116u |
| [16] |
E.R. Collantes, L. Xing, P.C. Miller, W.J. Welsh, S. Profeta, J. Agric. Food Chem. 47 (1999) 5245-5251. DOI:10.1021/jf9903410 |
| [17] |
M.Z. Wang, L. Zhang, X.W. Wang, et al., Chin. Chem. Lett. 29 (2018) 1375-1378. DOI:10.1016/j.cclet.2017.11.022 |
| [18] |
B.L. Wang, Y.X. Shi, Y. Ma, J. Agric. Food Chem. 58 (2010) 5515-5522. DOI:10.1021/jf100300a |
| [19] |
J.X. Chen, X.H. Gan, C.F. Yi, et al., Chin. J. Chem. 36 (2018) 939-944. DOI:10.1002/cjoc.201800282 |
| [20] |
X.J. Zou, L.H. Lai, G.Y. Jin, Z.X. Zhang, J. Agric. Food Chem. 50 (2002) 3757-3760. DOI:10.1021/jf0201677 |
 2020, Vol. 31
2020, Vol. 31