b CAS Key Laboratory of Chemistry of Northwestern Plant Resources/Key Laboratory for Natural Medicine of Gansu Province, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China;
c School of Life Sciences, Kyungpook National University, Daegu 41566, Republic of Korea
Small molecule biological thiols, such as cysteine (Cys), homocysteine (Hcy), and glutathione (GSH), play vital roles in many physiological and pathological processes [1, 2]. In the past decade, a variety of strategies have been developed for detecting bithiols, such as mass spectrometry, high-performance liquid chromatography (HPLC), capillary electrophoresis, electrochemical method and optical assays [3-5]. Compared to the optical assays, other methods required expensive analytical instruments, and cumbersome preconditioning procedures, and are not compatible with real-time analysis and intracellular assays. Thus, optical assays, especially the fluorescence sensing, have attracted increasing interests in biomedical researches, and a fruitful harvest of fluorescent probe has been achieved for biological small molecules, metal ions and enzymes [6-11]. Among the small molecule biological thiols, Cys is a fundamental sulfhydryl-containing amino acid in signal transduction and protein synthesis. The abnormal levels of Cys are closely related to many diseases, including edema, rheumatoid arthritis, cardiovascular diseases and liver damages [12-15]. Moreover, Cys has the similar structure and reaction activity compare to the Hcy and GSH. Thus, developed high selective and facile sensing strategies to discriminatively detect Cys are urgent.
So far, on the basis of different recognition mechanism and fluorescent signal groups, a series of fluorescent probes for Cys detection have been developed [16-20]. Despite these probes have tremendous advances, many probes utilized ultraviolet or visible-light as emissions. To the best of our knowledge, probes with short emission wavelength are not a good choice for biomedical imaging because of the potential interference triggered by short-wavelength light emission. To overcome this drawback, the fluorescent probe utilized the red and near-infrared emission fluorescent dyes as reported group with the merits of higher tissue penetration, low tissue photo-damage and little background interference become desired [21-25].
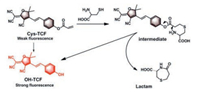
In this study, we constructed a novel fluorescent probe, termed as Cys-TCF, using OH-TCF (2-(4-(4-aminostyryl)-3-cyano-5, 5-dimethylfuran-2(5H)-ylidene)malono-nitrile), a red-emitting fluorophore with many applications [26, 27], as the platform and the acrylate group as a quencher of the fluorophore. The synthetic route of probe Cys-TCF was illustrated in Scheme S1 (Supporting information). The structure of all compounds was fully characterized by 1H NMR, 13C NMR and HRMS, and their original spectra were included in Figs. S1-S3 (Supporting information). The sulfhydryl group in Cys reacts with the electron-deficient double bond forming a thioether intermediate, which further experiences a spontaneous intermolecular cyclization to yield the fluorophore (OH-TCF). The proposed reaction processes were illustrated in Scheme 1.
|
Download:
|
| Scheme 1. The proposed mechanism of probe Cys-TCF to Cys. | |
With the Cys-TCF in hand, we explored the optical properties of Cys-TCF. Initially, the absorption spectrum of Cys-TCF was recorded, as shown in Fig. 1A. The probe shows a maximum absorption at 400 nm. After adding of Cys (50 μmol/L), the maximum absorption is red-shifted, which indicated that the conjugation between the OH-TCF and acrylate group is broken by thiol via nucleophilic substitution.

|
Download:
|
| Fig. 1. Response of Cys-TCF to Cys. (A) Time-dependent absorbance spectra of Cys-TCF towards Cys. The absorbance spectra were scanned every 1 min for 12 min. (B) Time-dependent emission spectra of Cys-TCF towards Cys. The inset shows the time-dependent changes of emission at 607 nm. (C) Dose-dependent emission spectra of Cys-TCF towards Cys. (D) Plotting the fold of fluorescence increase (F/F0) at 607 nm (λex = 530 nm) versus the concentrations of Cys. The inset shows F/F0 is linear to the concentrations of Cys in the range of 0.5–10 μmol/L. Data were expressed as mean ± standard deviation (SD) of three experiment. | |
We then investigated the fluorescence response of Cys-TCF after adding Cys, the result showed in Fig. 1B. The free Cys-TCF has a negligible fluorescence, after adding a certain amount of Cys (50 μmol/L), the fluorescent intensity of reaction mixture displays a rapid enhancement at 607 nm and reaches a plateau after 7 min, which suggested that a fast sensing response produce. Encouraged by the above result, the change of fluorescence intensity towards varying Cys concentrations (0 - 200 μmol/L) was also studied in detailed (Fig. 1C). The result displays a manner of Cys concentration dependence. The calibration curve in Fig. 1D shows linear relationship (R = 0.9961) of F/F0 towards the concentration of Cys. The limit of detection was found to be 0.04 μmol/L based on CDL = 3σ/k, where σ represents the standard deviation of the blank measurements, and k represents the linear regression slope.
It is well known that the application is crucial for fluorescent probe. To verify the practical application of Cys-TCF in living system, the fluorescence intensity change of probe towards Cys over a wide pH range (5.0–10.0) were investigated, as shown in Fig. S4 (Supporting information). The fluorescence signal increases with the increasement of pH from 5.0 to 7.4 and reaches a maximum at ~7.4 and maintained from 7.4 to 9.0. This result indicated that the Cys-TCF is suitable for detecting Cys under physiological condition. Due to the existence of abundant Hcy and GSH in living system, we further explored the selectivity of Cys-TCF toward the Cys. As shown in Fig. 2A, the addition of Cys (50 μmol/L) could trigger a stronger fluorescent enhancement, whereas Hcy, GSH and other amino acids give no response to the probe at higher concentration (1 mmol/L). This result demonstrates that the Cys-TCF possess high selectivity toward Cys and could be used to detect Cys in a complex sample. Then, the time-dependent changes of fluorescence intensity upon the reaction of Cys-TCF with Cys, Hcy and GSH were shown in Fig. 2B. The probe has weak fluorescence, while there was obvious fluorescent enhancement from 0~12 min and reached a plateau with the addition of Cys (50 μmol/L). In contrast, the Hcy and GSH give a slight response with Cys-TCF. Collectively, this probe could selectively recognize Cys in PBS buffer.

|
Download:
|
| Fig. 2. (A) Selective recognition of Cys by Cys-TCF. The fold of fluorescence increase at 607 nm (λex = 530 nm) was determined after mixing Cys-TCF with various analytes for 10 min in the PBS buffer, pH 7.4. The concentration of Cys was 100 μmol/L and the other analytes was 1 mmol/L. (B) Kinetics of the fluorescene change upon Cys-TCF responding to Cys, GSH and Hcy. The fold of fluorescence increase at 607 nm was determined after mixing Cys-TCF with Cys, GSH or Hcy for 10 min in the PBS buffer, pH 7.4. Data were expressed as mean ± SD of three experiments. | |
On the basis of previous studies [28] and above results, we hypothesized that Cys could react with the terminal acrylate group based on the cascade addition and release of the fluorophore via an intramolecular cyclization, thus a feasible reaction mechanism was proposed (Scheme 1). In order to verify the proposed mechanism, the reaction of Cys-TCF with Cys was further analyzed by HPLC and mass spectrometric analyses. As shown in Fig. S5 (Supporting information), the retention times of Cys-TCF and OH-TCF are 14.06 and 7.96 min, respectively. After the reaction of Cys-TCF with Cys, we found that the signal at 14.06 min disappeared and a signal at 7.81 min appeared. The retention time of new peak is consistent with the OH-TCF. This result verified the proposed mechanism that the Cys switched on fluorescence of the probe. Furthermore, the mass spectrometric (Fig. S6 in Supporting information) results also support the proposed mechanism.
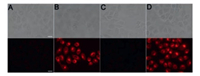
Cytotoxicity is a crucial factor for imaging applications. Firstly, we evaluated the cytotoxicity of Cys-TCF in HeLa cells by a conventional MTT assay as described in previous publications [29, 30]. The result indicates that the probe has very low cytotoxicity to the cells below 20 μmol/L and can be used for imaging applications (Fig. S7 in Supporting information). To further demonstrate the practical applications of Cys-TCF for biomedical imaging, we first carried out the fluorescence imaging in living cells. In the control group (Fig. 3A), the HeLa cells display no fluorescence signal. After the addition of Cys-TCF to the cultured HeLa cells, a strong red fluorescence signal was observed (Fig. 3B). It is worth noting that when the HeLa cell was pretreated with NEM, the thiol-blocking reagent, and then incubated with Cys-TCF (10 μmol/L) for another 30 min, the fluorescence intensity of probe was nearly unchanged. This was attributed to the blockage of the endogenous Cys by NEM (Fig. 3C). However, the group which added 100 μmol/L Cys in the NEM-pretreated HeLa cells and then incubated the cell with Cys-TCF for 30 min showed strong red fluorescence signal (Fig. 3D). These results indicated that the change of the fluorescence of the probe is due to its reaction with Cys. One of the outstanding advantages of red-emitting fluorescent probes is that they have low background signal in biological systems. We then applied the probe to image Cys in living zebrafishes. As shown in Fig. 4, the control fishes display nearly no fluorescence (Fig. 4A), while 3-day-old zebrafishes were exposed to Cys-TCF and showed strong red fluorescence (Fig. 4B). Moreover, a thiol-blocking experiment was performed to further investigate whether the fluorescence change of probe in zebrafishes results from Cys. Similarly, the slight fluorescence were observed in Fig. 4C, while Fig. 4D showed strong red fluorescence. The above results demonstrated that Cys-TCF is capable of monitoring the endogenous Cys in vivo.
|
Download:
|
| Fig. 3. Imaging Cys in live HeLa cells. (A) Control; (B) Image of the cell incubated with Cys-TCF (10 μmol/L) for 30 min; (C) Image of the cell treated with 1 mmol/L NEM for 30 min and subsequent treatment with Cys-TCF (10 μmol/L) for 30 min; (D) Image of the cell treated with 1 mmol/L NEM for 30 min, then treatment with Cys (200 μmol/L) and subsequent treatment with Cys-TCF (10 μmol/L) for 30 min. Scale bar: 25 μm. | |

|
Download:
|
| Fig. 4. Confocal images of Cys in zebrafishes. (A) Control; (B) Image of the zebrafishes incubated with Cys-TCF (10 μmol/L) for 30 min; (C) Image of the zebrafishes treated with 1 mmol/L NEM for 30 min and subsequent treatment with Cys-TCF (10 μmol/L) for 30 min; (D) Image of the zebrafishes treated with 1 mmol/L NEM for 30 min, then treatment with Cys (200 μmol/L) and subsequent treatment with Cys-TCF (10 μmol/L) for 30 min. The top panel is bright-field, the middle panel is fluorescence images from sample, and the bottom panel is merged images. | |
Comparisons of the proposed probe Cys-TCF with several other acrylate-functionalized fluorescent probes were summarized in Table S1 (Supporting information). These probes provide valuable tools for study of Cys [31-36]. However, some of the probes need UV excitation, which limits their biological applications [33, 37, 38]. Red or near-infrared (NIR) emission provides the advantages of higher tissue penetration, low tissue photo-damage and negligible background interference. Nevertheless, some of the red/NIR fluorescent probes suffer from long response time, or small Stokes shift [31, 32, 37, 39], while the proposed probe Cys-TCF possesses fast response, low detection limit and large Stokes shift. All these remarkable merits facilitate the biological applications of Cys-TCF in vitro and in vivo, and make it a powerful tool to study the biological functions of Cys.
In summary, a novel red-emitting fluorescence probe Cys-TCF has been rationally constructed for selective determination of Cys. This probe utilized the acrylate group as reaction site for Cys and has excellent sensitivity and selectivity from other structurally similar thiols and amino acids, such as Hcy, GSH. Additionally, the probe exhibits low detection limit for Cys and low cytotoxicity to HeLa cells. Furthermore, the experimental results of bioimaging in living cells and zebrafishes demonstrated that the Cys-TCF would have a promising prospect in detection of Cys, and we hope that this probe will be of great benefit for many biomolecules and bioimaging researchers.
AcknowledgmentsThe financial supports from the National Natural Science Foundation of China (Nos. 21708017, 21572093, 21778028), Lanzhou University (the Fundamental Research Funds for the Central Universities, No. lzujbky-2018-64), and Natural Science Foundation of Gansu Province (No. 17JR5RA193) are greatly acknowledged. The authors confirm that there are no conflicts of interest.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.05.061.
| [1] |
E. Weerapana, C. Wang, G.M. Simon, et al., Nature 468 (2010) 790-795. DOI:10.1038/nature09472 |
| [2] |
H. Zhang, J. Chen, Y. Yang, et al., Anal. Chem. 91 (2019) 5004-5010. DOI:10.1021/acs.analchem.8b04779 |
| [3] |
H.S. Jung, X. Chen, J.S. Kim, J. Yoon, Chem. Soc. Rev. 42 (2013) 6019-6031. DOI:10.1039/c3cs60024f |
| [4] |
L.Y. Niu, Y.S. Guan, Y.Z. Chen, et al., J. Am. Chem. Soc. 134 (2012) 18928-18931. DOI:10.1021/ja309079f |
| [5] |
A.R. Ivanov, I.V. Nazimov, L. Baratova, A.P. Lobazov, G.B. Popkovich, J. Chromatogr. A 913 (2001) 315-318. DOI:10.1016/S0021-9673(00)00993-6 |
| [6] |
W. Chyan, R.T. Raines, ACS Chem. Biol. 13 (2018) 1810-1823. DOI:10.1021/acschembio.8b00371 |
| [7] |
L. Zhang, S. Peng, J. Sun, et al., Chem. Sci. 8 (2017) 2966-2972. DOI:10.1039/C6SC04708D |
| [8] |
P. Zhou, J. Yao, G. Hu, J. Fang, ACS Chem. Biol. 11 (2016) 1098-1105. DOI:10.1021/acschembio.5b00856 |
| [9] |
L. Yang, H. Xiong, Y. Su, et al., Chin. Chem. Lett. 30 (2019) 563-565. DOI:10.1016/j.cclet.2018.12.017 |
| [10] |
M. Li, P. Cui, K. Li, et al., Chin. Chem. Lett. 29 (2018) 992-994. DOI:10.1016/j.cclet.2017.11.011 |
| [11] |
P. Ning, W. Wang, M. Chen, Y. Feng, X. Meng, Chin. Chem. Lett. 28 (2017) 1943-1951. DOI:10.1016/j.cclet.2017.09.026 |
| [12] |
X. Yang, Y. Guo, R.M. Strongin, Angew. Chem. Int. Ed. 50 (2011) 10690-10693. DOI:10.1002/anie.201103759 |
| [13] |
O. Rusin, Luce N.N.St., R.A. Agbaria, et al., J. Am. Chem. Soc. 126 (2004) 438-439. DOI:10.1021/ja036297t |
| [14] |
Y. Zhang, X. Shao, Y. Wang, et al., Chem. Commun. (Camb.) 51 (2015) 4245-4248. DOI:10.1039/C4CC08687B |
| [15] |
X. Jiao, Y. Li, J. Niu, et al., Anal. Chem. 90 (2018) 533-555. |
| [16] |
M. Zhang, L. Wang, Y. Zhao, et al., Anal. Chem. 90 (2018) 4951-4954. DOI:10.1021/acs.analchem.8b00682 |
| [17] |
Z.H. Fu, X. Han, Y. Shao, et al., Anal. Chem. 89 (2017) 1937-1944. DOI:10.1021/acs.analchem.6b04431 |
| [18] |
W. Niu, L. Guo, Y. Li, et al., Anal. Chem. 88 (2016) 1908-1914. DOI:10.1021/acs.analchem.5b04329 |
| [19] |
J. Chan, S.C. Dodani, C.J. Chang, Nat. Chem. 4 (2012) 973-984. DOI:10.1038/nchem.1500 |
| [20] |
Y. Yang, H. Wang, Y.L. Wei, et al., Chin. Chem. Lett. 28 (2017) 2023-2026. DOI:10.1016/j.cclet.2017.08.051 |
| [21] |
H. Zhou, Y. Xiao, X. Hong, Chin. Chem. Lett. 29 (2018) 1425-1428. DOI:10.1016/j.cclet.2018.08.009 |
| [22] |
W. Chen, S. Xu, J.J. Day, D. Wang, M. Xian, Angew. Chem. Int. Ed. 56 (2017) 16611-16615. DOI:10.1002/anie.201710688 |
| [23] |
Z. Guo, S. Park, J. Yoon, I. Shin, Chem. Soc. Rev. 43 (2014) 16-29. DOI:10.1039/C3CS60271K |
| [24] |
Z. Guo, S. Nam, S. Park, J. Yoon, Chem. Sci. 3 (2012) 2760-2765. DOI:10.1039/c2sc20540h |
| [25] |
P. Zhang, Z.Q. Guo, C.X. Yan, W.H. Zhu, Chin. Chem. Lett. 28 (2017) 1952-1956. DOI:10.1016/j.cclet.2017.08.038 |
| [26] |
S.J. Lord, N.R. Conley, H.-l.D. Lee, et al., J. Am. Chem. Soc. 130 (2008) 9204-9205. DOI:10.1021/ja802883k |
| [27] |
P. Gopalan, H.E. Katz, D.J. McGee, et al., J. Am. Chem. Soc. 126 (2004) 1741-1747. DOI:10.1021/ja039768k |
| [28] |
H. Wang, G. Zhou, H. Gai, X. Chen, Chem. Commun. 48 (2012) 8341-8343. DOI:10.1039/c2cc33932c |
| [29] |
X. Li, Y. Hou, X. Meng, et al., Angew. Chem. Int. Ed. 57 (2018) 6141-6145. DOI:10.1002/anie.201801058 |
| [30] |
D. Duan, J. Zhang, J. Yao, Y. Liu, J. Fang, J. Biol. Chem. 291 (2016) 10021-10031. DOI:10.1074/jbc.M115.700591 |
| [31] |
W. Zhang, J. Liu, Y. Yu, et al., Talanta 185 (2018) 477-482. DOI:10.1016/j.talanta.2018.04.001 |
| [32] |
Y. Qi, Y. Huang, B. Li, F. Zeng, S. Wu, Anal. Chem. 90 (2018) 1014-1020. DOI:10.1021/acs.analchem.7b04407 |
| [33] |
X. Dai, Q.H. Wu, P.C. Wang, et al., Biosens. Bioelectron. 59 (2014) 35-39. DOI:10.1016/j.bios.2014.03.018 |
| [34] |
J. Shi, Y. Wang, X. Tang, et al., Dyes Pigments 100 (2014) 255-260. DOI:10.1016/j.dyepig.2013.09.021 |
| [35] |
G. Liu, D. Liu, X. Han, et al., Talanta 170 (2017) 406-412. DOI:10.1016/j.talanta.2017.04.038 |
| [36] |
W. Fan, X. Huang, X. Shi, et al., Spectrochim. Acta A. Mol. Biomol. Spectrosc. 173 (2017) 918-923. DOI:10.1016/j.saa.2016.10.060 |
| [37] |
X. Yang, Y. Guo, R.M. Strongin, Org. Biomol. Chem. 10 (2012) 2739-2741. DOI:10.1039/c2ob25178g |
| [38] |
L. Wang, Q. Zhou, B. Zhu, et al., Dyes Pigments 95 (2012) 275-279. DOI:10.1016/j.dyepig.2012.05.006 |
| [39] |
D. Yu, Q. Zhang, S. Ding, G. Feng, RSC Adv. 4 (2014) 46561-46567. DOI:10.1039/C4RA06596D |
 2020, Vol. 31
2020, Vol. 31 

