b Shandong Province Key Laboratory of Detection Technology for Tumor Makers, School of Chemistry and Chemical Engineering, Linyi University, Linyi 276005, China;
c College of Chemistry and Materials Science, Nanning Normal University, Nanning 530001, China;
d Key Laboratory of Hunan Province for Water Environment and Agriculture Product Safety, Changsha 410083, China
Hydrazine (N2H4), a potentially versatile inorganic diamine with an ammonia-like smell, is widely used in many fields, including polymerization inhibitors, blowing agents, agricultural chemicals, rocket propellants, and anti-corrosion agents in water treatment [1-4]. Despite of its wide range of applications, N2H4 is highly toxic and dangerously unstable. Exogenous N2H4 can be readily absorbed and accumulated into human body through oral, dermal or breathing contact routes. In-depth studies on laboratory animals have revealed that endogenous N2H4is mutagenic and carcinogenic, which can cause critical damages to kidneys, lungs, liver, and central nervous system [5-9]. As a result, the World Health Organization and US Environmental Protect Agency have set a recommended threshold limit value (TLV) of N2H4 to be as low as 10 ppb (0.32 μmol/L) [4, 10]. Therefore, it is urgent and necessary to explore efficient analytical methods for sensitive and selective detection of hydrazine.
Conventional techniques for N2H4 detection such as flow detection [11], titrimetry[12], chromatography [13], potentiometry [14], electrochemical methods [15], chromatography-mass spectrometer [16], are usually subjected to expensive instrument, time-consuming, complicated sample preparation or relatively less sensitivity [17, 18]. Fluorescent probes were proved to be useful tools for qualitative and quantitative detection of various species both in vitro and in vivo with the advantages such as high sensitivity, good selectivity, non-invasion, easy operation and real-time imaging [19-23]. Currently, various fluorescent probes for the detection of N2H4 have been reported; however, most of them suffer from slow response time and insufficient sensitivity (Table S1 in Supporting information) [24-35]. Moreover, the concentration of N2H4 in vivo is low and rapidly fluctuates during the production and catabolism [36]. Therefore, a robust fluorescent probe with a quick response and a low detection limit is needed for the real-time detection of N2H4 in vivo.
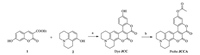
Coumarinocoumarin dyes are highly fluorescent and have a long-wavelength emission [37], which makes them a robust scaffold for the design of fluorescent probes. Herein, by incorporation of an acetate group to hydroxy julolidine-coumarinocoumarin (JCC), we have developed a novel fluorescent probe, JCCA, for the selective and sensitive detection of N2H4 (Scheme 1). We anticipated probe JCCA would be weakly fluorescent due to the photoinduced electron transfer effect from acetate group. Upon the treatment with N2H4, the ester bond in probe JCCA would be selectively cleaved to release a highly emissive dye JCC. The synthetic route of probe JCCA was shown in Scheme 1.
|
Download:
|
| Scheme 1. Synthetic route of probe JCCA. (a) No solvent, 120 ℃ for 2 h, yield 42%. (b) Acetic anhydride, 60 ℃ for 2 h, yield 54%. | |
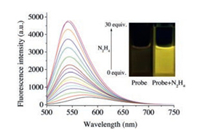
Before conducting fluorescence experiments, the solubility of probe JCCA in water (containing 5% DMSO) was measured to be 39.6 mg/L. The optical properties of probe JCCA in the absence/presence of N2H4 was investigated in PBS buffer (10.0 mmol/L, pH 7.4, containing 30% acetonitrile). Probe JCCA (10.0 μmol/L) exhibited an absorption maximum at 492 nm. Upon the treatment with excessive N2H4, the absorption at 492 nm disappeared and a new absorption peak at 473 nm emerged with a color change from pink to yellow (Fig. S1 in Supporting information). As expected, probe JCCA exhibited very weak fluorescence with λmaxem = 582 nm (Fig. 1). After the addition of N2H4, the solution of probe JCCA produced a strong yellow fluorescence with λmaxem = 542 nm. The intensities of the fluorescence signals at 542 nm gradually boosted up with increasing the concentration of N2H4 ranging from 0.0 μmol/L to 300.0 μmol/L. The maximal fluorescence enhancement was up to 21-fold. There was a good linearity (R = 0.996) between fluorescence intensity at 542 nm and the concentration of N2H4 in the range of 0.0–225.0 μmol/L (Fig. S2 in Supporting information). The detection limit was calculated to be 7.4 nmol/L (signal/noise = 3). Therefore, probe JCCA could be applied to qualitatively and quantitatively analyze N2H4 with excellent sensitivity. Dye JCC displayed an intensive absorption with an λmaxabs at 474 nm and was strongly fluorescent with an λmaxem at 542 nm. The similarity between the optical properties of dye JCC and the reaction mixture of probe JCCA with N2H4 evidently suggested that the addition of N2H4 to the solution of probe JCCA resulted in the formation of dye JCC (Scheme 2).
|
Download:
|
| Fig. 1. Fluorescence spectra of probe JCCA (10.0 μmol/L) in response to N2H4 (0.0–300.0 μmol/L) in PBS buffer (10.0 mmol/L, pH 7.4, containing 30% acetonitrile). Excitation wavelength: 480 nm. Excitation and emission slits (nm): 5.0/5.0. Inset: photos of the solution of probe JCCA in the absence/presence of N2H4. | |

|
Download:
|
| Scheme 2. Proposed sensing mechanism of probe JCCA towards N2H4. | |
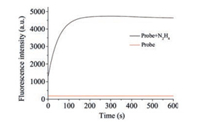
Time-dependent fluorescence experiments were carried out by treatment of the solution of probe JCCA (10.0 μmol/L) with N2H4 (30 equiv.) in PBS buffer (10.0 mmol/L, pH 7.4, containing 30% acetonitrile). As seen in Fig. 2, the addition of N2H4 instantaneously induced strong fluorescent signals, which were maximized within 3 min. In contrast, little fluorescence change was observed when the solution of probe JCCA was in the absence of N2H4 during the same interval. These results suggested probe JCCA was stable and exhibited a quick response toward to N2H4.
|
Download:
|
| Fig. 2. Time-dependent fluorescence experiment of probe JCCA (10.0 μmol/L) in the absence and presence of N2H4 (300.0 μmol/L) in PBS buffer (10 mmol/L, pH 7.4, containing 30% acetonitrile). | |
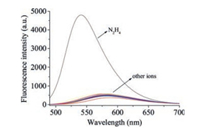
To evaluate the selectivity of probe JCCA for N2H4, the fluorescence responses of probe JCCA towards common relevant species (30.0 equiv.) were determined, including biothiols (Cys, Hcy, GSH), cations (Na+, K+, Mg2+, Al3+, Zn2+, Cu2+, Ca2+, Hg2+, Fe2+), anions (N3-, F-, Cl-, I-, Br-, CO32-, PO42-, SO42-, S2-, SCN-, NO3-, AcO-) and nitrogen-containing compounds (aniline, thiourea, urea, NH2OH). As shown in Fig. 3, the fluorescence spectra of probe JCCA exhibited negligible changes in the presence of these relevant analytes. Meanwhile, the co-existence of these relevant species caused no interference on the performance of probe JCCA for the detection of N2H4 (Fig. S3 in Supporting information). All the above results clearly showed that probe JCCA had a good selectivity toward N2H4.
|
Download:
|
| Fig. 3. Fluorescence spectra of probe JCCA (10.0 μmol/L) upon the addition of N2H4 and other analytes (300.0 μmol/L) in PBS buffer (10 mmol/L, pH 7.4, containing 30% acetonitrile). | |
In order to evaluate whether probe JCCA can function well in biological samples, we investigated its fluorescence property in the absence/present of N2H4 in the media with different pH values (Fig. S4 in Supporting information). In the absence of N2H4, the fluorescence intensity hardly changed in a pH range between 2.0-10.0. In the presence of N2H4 (30.0 equiv.), the solution of probe JCCA displayed strong fluorescent signals with a maximum at 542 nm in a pH range of 7.0-10.0. Therefore, it could be concluded that probe JCCA had a good performance in the detection of N2H4 under physiological condition.
Finally, the potential application of probe JCCA in living cells was carried out (Fig. 4). First, the cytotoxicity of probe JCCA was evaluated using HeLa cells by the methyl thiazolyl tetrazolium (MTT) assay. A 96% cell viability was obtained when cells were incubated with 10.0 μmol/L of probe JCCA for 24 h, indicating probe JCCA was non-toxic (Fig. S5 in Supporting information). When HeLa cells were incubated with probe JCCA (10.0 μmol/L) for 30 min, very weak orange fluorescence (bandpass filter 560–600 nm) was observed (Fig. 4d). When HeLa cells were incubated with probe JCCA (10.0 μmol/L) for 30 min and then treated with N2H4(300.0 μmol/L) for another 30 min, bright yellow fluorescent signals (bandpass filter 520–560 nm) occurred from cells (Fig. 4a).

|
Download:
|
| Fig. 4. Fluorescence (left column), bright field (middle column) and merged (right column) images of HeLa cells. Top row: cells incubated with probe JCCA (10.0 μmol/L) for 30 min and subsequently treated with N2H4 (300.0 μmol/L) for another 30 min. Bottom row: cells incubated with probe JCCA (10.0 μmol/L) for 30 min. Yellow channel (520–560 nm, excited at 488 nm); Orange channel (560–600 nm, excited at 488 nm). | |
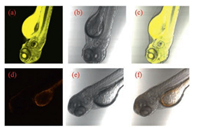
Furthermore, we used three-day old zebra fish to investigate the capability of probe JCCA to detect N2H4 in living organisms (Fig. 5). When zebra fish was incubated with probe JCCA (10.0 μmol/L) for 30 min at 28 ℃, and then treated with N2H4(300.0 μmol/L) for another 30 min, strong yellow fluorescent signals (bandpass filter 560–600 nm) were seen from zebra fish (Fig. 5a). In contrast, the incubation of zebra fish with probe JCCA (10.0 μmol/L) only resulted in weak orange fluorescence (bandpass filter 560–600 nm) (Fig. 5d).
|
Download:
|
| Fig. 5. Fluorescence (left column), bright field (middle column) and merged (right column) images of three-day old zebra fish. Top row: zebra fish incubated with probe JCCA (10.0 μmol/L) and then treated with N2H4 (300.0 μmol/L). Bottom row: zebra fish incubated with probe JCCA(10.0 μmol/L). Yellow channel (520–560 nm, excited at 488 nm); Orange channel (560–600 nm, excited at 488 nm). | |
In conclusion, we developed a new fluorescent probe for the detection of N2H4 on the basis of a coumarinocoumarin derivative. This probe displayed a fast response time with high sensitivity, good selectivity, and a low detection limit (7.4 nmol/L). The application of this probe in living cells and organism demonstrated it had a good potential for the detection of N2H4 in biological systems.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. U1608222), Special Fund for Agro-scientific Research in the Public Interest of China (No. 201503108), the State Key Laboratory of Fine Chemicals (No. KF1606) and the State Key Laboratory of Chemo/Biosensing and Chemometrics (No. 2016005).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.021.
| [1] |
J. Sanabria-Chinchilla, K. Asazawa, T. Sakamoto, et al., J. Am. Chem. Soc. 133 (2011) 5425-5431. DOI:10.1021/ja111160r |
| [2] |
A. Serov, M. Padilla, A.J. Roy, et al., Angew. Chem. Int. Ed. 53 (2014) 10336-10339. DOI:10.1002/anie.201404734 |
| [3] |
A.D. Sutton, A.K. Burrell, D.A. Dixon, et al., Science 331 (2011) 1426-1429. DOI:10.1126/science.1199003 |
| [4] |
S.D. Zelnick, D.R. Mattie, P.C. Stepaniak, Aviat. Space Envir. MD. 74 (2003) 1285-1291. |
| [5] |
S. Garrod, M.E. Bollard, A.W. Nicholls, et al., Chem. Res. Toxicol. 18 (2005) 115-122. DOI:10.1021/tx0498915 |
| [6] |
G. Wang, C. Zhang, X. He, et al., Electrochim. Acta 55 (2010) 7204-7210. DOI:10.1016/j.electacta.2010.07.053 |
| [7] |
H. Yang, B. Lu, L. Guo, B. Qi, J. Electroanal. Chem. 650 (2011) 171-175. DOI:10.1016/j.jelechem.2010.10.018 |
| [8] |
X. Zhang, C. Shi, P. Ji, et al., Anal. Methods 8 (2016) 2267-2273. DOI:10.1039/C5AY03313F |
| [9] |
J. Liu, J. Shen, M. Li, L.P. Guo, Chin. Chem. Lett. 26 (2015) 1478-1484. DOI:10.1016/j.cclet.2015.10.026 |
| [10] |
X. Xia, F. Zeng, P. Zhang, et al., Sensor. Actuat. B-Chem. 227 (2016) 411-418. DOI:10.1016/j.snb.2015.12.046 |
| [11] |
Z.K. He, B. Fuhrmann, U. Spohn, Anal. Chim. Acta 409 (2000) 83-91. DOI:10.1016/S0003-2670(99)00890-9 |
| [12] |
H.E. Malone, D.M.W. Anderson, Anal. Chim. Acta 48 (1969) 87-91. DOI:10.1016/S0003-2670(01)85244-2 |
| [13] |
P.E. Kester, N.D. Danielson, Chromatographia 18 (1984) 125-128. DOI:10.1007/BF02258767 |
| [14] |
P. Bravo, F. Isaacs, G. Ramírez, et al., J. Coord. Chem. 60 (2007) 2499-2507. DOI:10.1080/00958970701275790 |
| [15] |
A. Benvidi, P. Kakoolaki, H.R. Zare, R. Vafazadeh, Electrochim. Acta 56 (2011) 2045-2050. DOI:10.1016/j.electacta.2010.11.083 |
| [16] |
M. Sun, L. Bai, D.Q. Liu, J. Pharm. Biomed. Anal. 49 (2009) 529-533. DOI:10.1016/j.jpba.2008.11.009 |
| [17] |
X. Dai, Z.Y. Wang, Z.F. Du, J.Y. Miao, B.X. Zhao, Sensor. Actuat. B-Chem. 232 (2016) 369-374. DOI:10.1016/j.snb.2016.03.159 |
| [18] |
Y. Ding, S. Zhao, Q. Wang, X. Yu, W. Zhang, Sensor. Actuat. B-Chem. 256 (2018) 1107-1113. DOI:10.1016/j.snb.2017.10.119 |
| [19] |
Y. Gao, Y. Lin, T. Liu, et al., Chin. Chem. Lett. 30 (2019) 63-66. DOI:10.1016/j.cclet.2018.03.028 |
| [20] |
Y. Geng, H. Tian, L. Yang, X. Liu, X. Song, Sensor. Actuat. B-Chem. 273 (2018) 1670-1675. DOI:10.1016/j.snb.2018.07.088 |
| [21] |
L. Yu, Y. Qiao, L. Miao, Y. He, Y. Zhou, Chin. Chem. Lett. 29 (2018) 1545-1559. DOI:10.1016/j.cclet.2018.09.005 |
| [22] |
W. Qin, C. Xu, Y. Zhao, et al., Chin. Chem. Lett. 29 (2018) 1451-1455. DOI:10.1016/j.cclet.2018.04.007 |
| [23] |
Z. Xu, X. Huang, X. Han, et al., Chem 4 (2018) 1609-1628. DOI:10.1016/j.chempr.2018.04.003 |
| [24] |
Y. Hao, Y. Zhang, K. Ruan, et al., Spectrochim. Acta A 184 (2017) 355-360. DOI:10.1016/j.saa.2017.04.041 |
| [25] |
C. Hu, W. Sun, J. Cao, et al., Org. Lett. 15 (2013) 4022-4025. DOI:10.1021/ol401838p |
| [26] |
Z. Ju, D. Li, D. Zhang, et al., J. Fluoresc. 27 (2017) 1-9. DOI:10.1007/s10895-016-1930-0 |
| [27] |
X. Kong, B. Dong, C. Wang, et al., J. Photochem. Photobiol. A 356 (2018) 321-328. DOI:10.1016/j.jphotochem.2018.01.009 |
| [28] |
Y. Qian, J. Lin, L. Han, L. Lin, H. Zhu, Biosens. Bioelectron. 58 (2014) 282-286. DOI:10.1016/j.bios.2014.02.059 |
| [29] |
Y.Z. Ran, H.R. Xu, K. Li, et al., RSC Adv. 6 (2016) 111016-111019. DOI:10.1039/C6RA24110G |
| [30] |
X. Shi, F. Huo, J. Chao, C. Yin, Sensor. Actuat. B-Chem. 260 (2018) 609-616. DOI:10.1016/j.snb.2017.12.202 |
| [31] |
Y. Sun, D. Zhao, S. Fan, L. Duan, Sensor. Actuat. B-Chem. 208 (2015) 512-517. DOI:10.1016/j.snb.2014.11.057 |
| [32] |
H. Tse, Q. Li, S. Chan, et al., RSC Adv. 6 (2016) 14678-14681. DOI:10.1039/C5RA26683A |
| [33] |
W. Xu, W. Liu, T. Zhou, Y. Yang, W. Li, J.Photochem. Photobiol. A 356 (2018) 610-616. DOI:10.1016/j.jphotochem.2018.02.004 |
| [34] |
J. Zhang, L. Ning, J. Liu, et al., Anal. Chem. 87 (2015) 9101-9107. DOI:10.1021/acs.analchem.5b02527 |
| [35] |
T. Tang, Y.Q. Chen, B.S. Fu, et al., Chin. Chem. Lett. 27 (2016) 540-544. DOI:10.1016/j.cclet.2016.01.024 |
| [36] |
E.W. Schmidt, Hydrazine and Its Derivatives: Preparation, Properties, Applications, 2nd ed., Wiley-Interscience, 2001.
|
| [37] |
C. Chen, L. Zhou, W. Liu, W. Liu, Anal. Chem. 90 (2018) 6138-6143. DOI:10.1021/acs.analchem.8b00434 |
 2020, Vol. 31
2020, Vol. 31 

