b State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Shanghai 200237, China
Alkaline phosphatase (ALP) exists in mammalian tissues and mediates dephosphorylation of biomolecules [1, 2]. The abnormal fluctuation of the ALP concentration in serum has been established as an important biomarker for diagnosis of such diseases as hepatobiliary disease, bone disease associated with increased osteoplastic activity, breast cancer and prostate cancer [3, 4]. Therefore, a rapid and reliable assay against ALP can facilitate disease diagnosis in clinical settings.
Fluorescence based techniques are versatile and valuable for analysis and imaging of the biomolecules in vitro or in vivo studies because of its high sensitivity and feasibility for direct use in complex milieu. For the ALP fluorescent sensing, various synthetic probes have been reported for the detection and the activity monitoring of the ALP [5-22] which were typically designed by modification of the electron donating group of a fluorescent dye and the fluorescent signals were generated by hydrolyzing the phosphate group of the probe. However, such probes are usually limited in that the fluorescence emission wavelengths of the probe and the product are not well separated so that the signal from the probe constitutes a background, which compromises the detection of low concentration of ALP. Therefore, we wish to report a novel probe which totally eliminates the background signal and render sensitive and accurate detection of ALP especially of low concentration. Excited-state intramolecular proton transfer (ESIPT) is a well-known photochemical process, which allows an unusual large stokes shifts during the alteration the conjugation system of the fluorophore [23]. It has been gained much attention in the fluorescence sensing of various substrates such as hydrazine [24], H2O2 [25], phosphatase MKP-6 [26] and superoxide [27]. Several ESIPT based fluorescent probes for ALP detection have been reported recently. Tang and coworkers presented a ratiometric probe based on a phosphorylated chalcone derivative with AIE characteristics and ESIPT performances, enabling a detection limit as low as 0.15 mU/mL [15]. Another ESIPT probe for ALP was reported by Fan and co-workers, however, in which the red-shift wavelength was only 70 nm and the detection limit reached 1.3 mU/mL [28]. Until now, the lowest detection limit of ALP has reached 0.035 mU/mL, however, it was developed by employing the copper nanoparticle [29]. The reported lowest detection limit of ALP from small fluorescence probe is 0.0077 mU/mL [30].
The fluorophore N-(3-(benzo[d]thiazol-2-yl)-4-hydroxyphenyl)benzamide (BTHPB) has been reported as an excellent fluorescent molecule based on ESIPT [31], which exhibits two emission bands originating from enol and keto forms at 425 nm and 545 nm, respectively [32, 33]. The two emissions are well separated by nearly 120 nm, which is sufficient to allow the signals of two bands non-overlapping. Based on this molecule, fluorescent sensors for fluoride, palladium species and mercury, in which all of them exhibited extreme low detection limit [34-36]. Therefore, in this work, we present an ALP probe 1 (4-benzamio-2-(benzo[d]thiazol-2-yl)phenyl dihydrogen phosphate, Scheme 1) by employing BTHPB as the fluorescent reporting molecule. We coupled phosphate group with BTHPB together to obtain a more sensitive fluorescent probe 1 which enables "turn-on" ratiometric fluorescence signal toward ALP addition.

|
Download:
|
| Scheme 1. (A) The sensing mechanism of ALP by using probe 1. The phosphate group was released because of ALP catalysis, resulting the BTHPB. (B) Synthesis route of the fluorophore BTHPB and the probe 1 for ALP sensing. Reagents and conditions: (a) polyphosphoric acid, 180 ℃, 3 h, yield 49%; (b) benzoyl chloride, pyridine, 0 ℃ – r.t., 8 h, yield 23%; (c) (EtO)2POCl, t-BuOK, THF, r.t., overnight, yield 43%; (d) BSTFA, (CH3)3SiI, CH2Cl2, 0 ℃ – r.t., 1 h; (e) HCl, H2O/CH3CN, r.t., 30 min, HPLC, yield 64%. | |
As illustrated in Scheme 1A, probe 1 was designed by transforming the hydroxyl group of BTHPB into the corresponding phosphate group and the benzoamide group could reduce the pH interference of the probe as well. In the presence of ALP, probe 1 was hydrolyzed and the BTHPB was released, which exhibited large emission spectral red-shift (∼120 nm) because of the recovery of ESIPT process. The synthesis of probe 1 was shown in Scheme 1B, which was obtained by a four-step synthesis [37] and the structure was confirmed by 1H NMR, 13C NMR and HR—MS. Besides, the UV–vis absorption and the fluorescence spectra of the probe 1 and the hydrolysis product (BTHPB) were investigated (Fig. S1 in Supporting information). It has been known that the ALP is most stable in the pH range 7.5–9.5 [38] and the pH optimum for the enzyme activity is pH 8–10 [39]. First, the spectral studies of the pH titration were carried out by investing the absorbance change and fluorescence intensity separately (Fig. S2 in Supporting information). The absorbance decreased when the pH value below 6.5 (Fig. S2a), which mainly because of the intramolecular hydrogen bond change between the phosphonate group and benzothiazole moiety in the probe. The fluorescence intensity gradually increased at 425 nm by changing the pH value from 1 to 7 and reached a stable value from pH 7 to pH 12. Therefore pH 8.0 is suitable for measuring the ALP activity in this work. Besides, the quantum yield of probe 1 was calculated to be 0.028 by using quinine sulfate in sulfuric acid as the reference.
Fig. 1a demonstrates the kinetic studying of dephosphorylation reation by the titration of ALP into probing solution. The ALP (1 mU/mL) was added into a solution of probe 1 (5 μmol/L) in Tris buffer (20 μmol/L, pH 8.0) containing 0.01% of Triton-X with 1% DMSO as a co-solvent. The fluorescence emission spectra were recorded at 5 min intervals over 60 min with excitation at 340 nm. In Fig. 1a, there is a dramatic fluorescence emission intensity enhancement at the wavelength of 545 nm while another fluorescence signal decrement occurred at the wavelength of 425 nm. The quantum yield of the BTHPB reached 0.16 after the enzymatic reaction. The wavelength red-shift is as much as 120 nm and there is almost no overlap between two emission bands. From the inset data in Fig. 1a, it can be seen that the fluorescent signal tended to be stable in the last 15 min, indicating that the enzyme reaction was almost completed in 45 min. Moreover, the peak intensities at 545 nm and 425 nm varied as an exponential or logarithmic function with the reaction time, respectively. And the ratiometric fluorescent plot (I545/I425) of probe 1 exhibited an excellent linear relationship within reaction time of 35 min (Fig. 1b). No matter the fluorescence intensity vs. time or the fluorescent ratio change vs. time, the results indicated that the probe 1 might be applied not only for the ALP sensing but also for the further kinetic analysis of ALP. Meanwhile, ALP-enzymatic reaction was examined by employing high performance liquid chromatography (HPLC) (Fig. S3 in Supporting information). Same retention time of the hydrolyzing product and the BTHPB was observed, indicating that the BTHPB was produced after the addition of ALP as expected.

|
Download:
|
| Fig. 1. Fluorescent assay of the dephosphorylated reaction of probe 1 at 24 ℃. (a) The time-dependent fluorescent intensities of probe 1 reacted with ALP (1 mU/mL) were recorded under certain intervals for 60 min. Inset: plot of peak intensities (orange dot) at 545 nm and (blue triangle) at 425 nm vs. reaction time. (b) Ratiometric fluorescence response (I545/I425) and the linear fit of probe 1 in 35 min. (c) Fluorescence spectra of probe 1 after the incubation with the different concentration of ALP for over 1 h at 24 ℃. (d) Fluorescence emission ratio (I545/I425) of probe 1 vs. concentration of ALP. The reaction buffer: 20 mmol/L Tris-HCl, pH 8.0, 0.01% Trition-X, 1% DMSO; probe 1: 5 μmol/L; excitation wavelength: 340 nm. | |
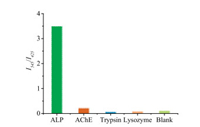
Furthermore, the quantitative analysis of ALP activity was examined. Fluorescence spectra were recorded after incubation of different amounts of ALP with probe 1 of same concentration (5 μmol/L) for over 1 h. Besides, the fluorescence spectrum of a solution containing BTHPB of 5 μmol/L was also collected as the control. As shown in Figs. 1c and d, the addition of ALP resulted in the dramatic fluorescence emission intensity enhancement at wavelength of 545 nm. Moreover, it can be seen that 2 mU/mL ALP can fully hydrolyze the probe 1 to BTHPB to reach almost the same fluorescence intensity by taking the fluorescence intensity of BTHPB as the contrast. In Fig. 1c, the emission ratio (I545/I425) of probe 1 varied from 0.145 to 17.76 upon the concentration of ALP increased from 0.1 mU/mL to 0.4 mU/mL, a ca. 122-fold enhancement was obtained, demonstrating that probe 1 has extreme high sensitivity. Meanwhile, the emission ratio also exhibited a linear relationship with ALP activity under the low concentration range (Fig. 1d). The detection limit of probe 1 to ALP was calculated and determined to be 0.004 mU/mL (S/N = 3, Fig. S4 in Supporting information). Besides, we also examined the selectivity performances of probe 1 over a series of enzymes including acetylcholinesterase (AChE), trypsin and lysozyme. The results were illustrated in Fig. 2. It can be observed that the probe 1 exhibited excellent selectivity of ALP over other enzymes while other three enzymes showed negligible fluorescence changes under the same conditions.
|
Download:
|
| Fig. 2. Fluorescent intensity ratio (I545/I425) changes of probe 1 (5 μmol/L) in the presence of ALP, AChE, trypsin and lysozyme (0.15 mU/mL). The reaction buffer: 20 mmol/L Tris-HCl, pH 8.0, 0.01% Trition-X, 1% DMSO (for lysozyme the pH value is 6.5); excitation wavelength: 340 nm; reaction time: 90 min. | |
Furthermore, we monitored the endogenous ALP activity in living cell by adding the probe to HeLa cells, of which the ALP was overexpressed. We first examined cytotoxicity of probe 1 by MTT assay. As shown in Fig. 3, there was almost no reduction in cell viability after the cells were incubated with the probe (from 5 μmol/L to 20 μmol/L), demonstrating that the probe has low toxicity. Then the imaging of endogenous ALP of HeLa cells was investigated by incubating the cells with probe 1 and the results were all illustrated in Fig. 4. By comparing with the control test (Fig. 4b), Fig. 4d showed fluorescence enhancement under the UV excitation, demonstrating the existence of endogenous ALP in HeLa cells and the generation of dephosphorylation product of probe 1. After the cells were pre-incubated with ALP inhibitor levamisole hydrochloride for 10 min and then the probe 1 was added, it was observed that the yellow fluorescence decreased (Fig. 4f), indicating that our probe can specifically probe overexpressed endogenic ALP in living cells.

|
Download:
|
| Fig. 3. Cell viability assay of probe 1 on HeLa cells. | |

|
Download:
|
| Fig. 4. Fluorescence images of HeLa cells in the absence or in the presence of probe 1. (a, c, e) Bright field images corresponding to the fluorescence images with (b) no probe 1, (d) probe 1 (5 μmol/L) and (f) inhibitor levamisole hydrochloride (5 mmol/L) and probe 1 (5 μmol/L). Excitation wavelength: 330–400 nm. Scale bar: 50 μm. | |
In summary, we designed and synthesized a fluorescent ratiometric probe for alkaline phosphatase detection based on the ESIPT mechanism. The probe was hydrolyzed by the ALP and the ESIPT fluorophore was produced, which accomplished a large red-shift (120 nm) of the fluorescent emission wavelength from 425 nm to 545 nm. The ratiometric value of two emissions vs. the low concentration of the ALP exhibited an excellent linear relationship, demonstrating that our probe is suitable for quantitative analysis of ALP especially at low concentrations. The probe exhibits extreme high sensitivity to the ALP, the low detection limit is as low as 0.004 mU/mL, which is quite outstanding compared with the reported "turn-on" fluorescent sensors and provides an efficient approach for the ALP analyzing. The fluorescent probe could also be applied for probing the biogenic ALP directly in living cells, which provides an excellent tools for quick detection and imaging biomarkers and shows potential applications in future point-of-care diagnosis.
AcknowledgmentsThis work was supported by the Shanghai Sailing Program, National Natural Science Foundation of China (No. 31600802), Science and Technology Commission of Shanghai Municipality (No. 18DZ1112703) and the Fundamental Research Funds for the Central Universities.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.037.
| [1] |
J.L. Millán, Mammalian Alkaline Phosphatases: FromBiology to Applications in Medicine and Biotechnology, Wiley-VCH, Weinheim, 2006.
|
| [2] |
R.B. McComb, G.N. Bowers, S. Posen, Alkaline Phosphatase, Plenum Press, New York, 1979.
|
| [3] |
G. Ramaswamy, V.R. Rao, L. Krishnamoorthy, et al., Indian J. Clin. Biochem. 15 (2000) 110-113. |
| [4] |
D. Goltzman, D. Miao, Encyclopedia Endocr. Dis. 1 (2004) 164-169. |
| [5] |
Z. Xu, X. Huang, X. Han, et al., Chem 7 (2018) 1609-1628. |
| [6] |
W. Chen, Y. Pan, J. Chen, et al., Chin. Chem. Lett. 29 (2018) 1429-1435. DOI:10.1016/j.cclet.2018.08.011 |
| [7] |
X Han, Y. Liu, G. Liu, et al., Chem. -Asian J. 14 (2019) 890-895. DOI:10.1002/asia.201801854 |
| [8] |
Z. Xu, J. Chen, L.L. Hu, et al., Chin. Chem. Lett. 28 (2017) 1935-1942. DOI:10.1016/j.cclet.2017.07.018 |
| [9] |
Z. Xu, L. Xu, J. Zhou, et al., Chem. Commun. (Camb.) 48 (2012) 10871-10873. DOI:10.1039/c2cc36141h |
| [10] |
B. Li, Z. He, H. Zhou, H. Zhang, T. Cheng, Chin. Chem. Lett. 28 (2017) 1929-1934. DOI:10.1016/j.cclet.2017.08.055 |
| [11] |
J. Zhu, P. Jia, N. Li, et al., Chin. Chem. Lett. 29 (2018) 1445-1450. DOI:10.1016/j.cclet.2018.09.002 |
| [12] |
D. Yue, M. Wang, F. Deng, et al., Chin. Chem. Lett. 29 (2018) 648-656. DOI:10.1016/j.cclet.2018.01.046 |
| [13] |
J. Park, Y. Kim, Bioorg. Med. Chem. Lett. 23 (2013) 2332-2335. DOI:10.1016/j.bmcl.2013.02.063 |
| [14] |
H. Gong, G. Little, M. Cradduck, et al., Talanta 84 (2011) 941-946. DOI:10.1016/j.talanta.2011.02.035 |
| [15] |
Z.G. Song, R.T.K. Kwok, E.G. Zhao, et al., ACS Appl. Mater. Interfaces 6 (2014) 17245-17254. DOI:10.1021/am505150d |
| [16] |
X.B. Wang, Z.Y. Zhang, X.Y. Ma, et al., Talanta 137 (2015) 156-160. DOI:10.1016/j.talanta.2015.01.028 |
| [17] |
X. Zhou, Y. Jiang, X. Zhao, Y. Zhu, Molecules 21 (2016) 1619-1628. DOI:10.3390/molecules21121619 |
| [18] |
M.H. Lee, J.S. Kim, J.L. Sessler, Chem. Soc. Rev. 44 (2015) 4185-4191. DOI:10.1039/C4CS00280F |
| [19] |
Y. Tan, L. Zhang, K. Man, et al., ACS Appl. Mater. Interfaces 9 (2017) 6796-6803. DOI:10.1021/acsami.6b14176 |
| [20] |
C. Chen, J. Zhao, Y. Lu, J. Su, X. Yang, Anal. Chem. 90 (2018) 3505-3511. DOI:10.1021/acs.analchem.7b05325 |
| [21] |
P. Ou, R. Zhang, Z. Liu, et al., Angew. Chem. Int. Ed. 58 (2019) 2261-2265. DOI:10.1002/anie.201811391 |
| [22] |
H. Wang, Z. Feng, S.J.D. Signore, A.A. Rodal, B. Xu, J. Am. Chem. Soc. 140 (2018) 3505-3509. DOI:10.1021/jacs.7b13307 |
| [23] |
J. Zhao, S. Ji, Y. Chen, H. Guo, P. Yang, Phys. Chem. Chem. Phys. 14 (2012) 8803-8817. DOI:10.1039/C2CP23144A |
| [24] |
S. Goswami, S. Das, K. Aich, et al., Org. Lett. 15 (2013) 5412-5415. DOI:10.1021/ol4026759 |
| [25] |
G. Li, D. Zhu, Q. Liu, L. Xue, H. Jiang, Org. Lett. 15 (2013) 924-927. DOI:10.1021/ol4000845 |
| [26] |
T. Kim, H. Kang, G. Han, S. Chung, Y. Kim, Chem. Commun. 2 (2009) 5895-5897. |
| [27] |
D. Murale, H. Kim, W. Choi, D. Churchill, Org. Lett. 15 (2013) 3946-3949. DOI:10.1021/ol4017222 |
| [28] |
C. Fan, S. Luo, H. Qi, Luminescence 31 (2016) 423-427. DOI:10.1002/bio.2977 |
| [29] |
J. Li, L. Si, J. Bao, Z. Wang, Z. Dai, Anal. Chem. 89 (2017) 3681-3685. DOI:10.1021/acs.analchem.6b05112 |
| [30] |
W. Zhang, H. Yang, N. Li, N. Zhao, RSC Adv. 8 (2018) 14995-15000. DOI:10.1039/C8RA01786G |
| [31] |
R. Hu, S. Li, Y. Zeng, et al., Phys. Chem. Chem. Phys. 13 (2011) 2044-2051. DOI:10.1039/C0CP01181A |
| [32] |
S. Li, Q. Wang, Y. Qian, et al., J. Phys. Chem. A 111 (2007) 11793-11800. DOI:10.1021/jp075301a |
| [33] |
S.R. Grando, C.M. Pessoa, M.R. Gallas, et al., Langmuir 25 (2009) 13219-13223. DOI:10.1021/la902242y |
| [34] |
R. Hu, J. Feng, D. Hu, et al., Angew. Chem. Int. Ed. 49 (2010) 4915-4918. DOI:10.1002/anie.201000790 |
| [35] |
L. Cui, W. Zhu, Y. Xu, X. Qian, Anal. Chim. Acta 786 (2013) 139-145. DOI:10.1016/j.aca.2013.05.011 |
| [36] |
M. Santra, B. Roy, K.H. Ahn, Org. Lett. 13 (2011) 3422-3425. DOI:10.1021/ol2011693 |
| [37] |
E. Barni, P. Savarino, M. Marzona, M. Piva, J. Heterocycl. Chem. 20 (1983) 1517-1521. DOI:10.1002/jhet.5570200616 |
| [38] |
M. Fosset, D. Chappelet-Tordo, M. Lazdunski, Biochemistry 13 (1974) 1783-1788. DOI:10.1021/bi00706a001 |
| [39] |
H.N. Fernley, P.D. Boyer (Eds.), The Enzymes, Vol. 4, Academic Press, New York, 1971, pp. 417-447.
|
 2020, Vol. 31
2020, Vol. 31 

