b School of Technology and Physical Science, ShanghaiTech University, Shanghai 201210, China
N-Methylation of amines is one of the most important synthetic reactions of fine chemicals, agricultural chemicals, drugs and dyes [1, 2]. N-Methyl amines are a structural subunit that can be found in many significant bioactive compounds and drugs [3, 4]. In particular, the lipid solubilityand permeability in human bodies for pharmaceuticals can be improved upon the methylation [5, 6]. In Fig. 1, some examples of important bioactive compounds containing N-methyl subunit are shown.

|
Download:
|
| Fig. 1. Several important compounds containing N-methyl moieties in their structure. | |
Classical methods to install N-methyl group include direct N-methylation using iodomethane or methyl sulfate and Eschweiler-Clarke reaction, where formaldehyde serves as the C1 source. Replacement of these toxic classical methylation agents by safer alternatives has been long pursued. Formic acid, methanol [7] and CO2 has recently emerged as new methylation agents. Formic acid is a cheap, readily available chemical with wide industrial applications, such as pesticide, medicine, printing and dyeing [8], and emerging application as a hydrogen storage chemical [9, 10]. Formic acid can be prepared from hydrogenation [11-13] or electrochemical reduction [14] of CO2. As such, methylation with formic acid as the C1 source is often considered as an indirect utilization of CO2. However, compared to the intense research efforts devoted to CO2 as the methylation agent [15, 16], much less progress has been achieved for the counterpart using formic acid.
Both homogeneous and heterogeneous catalyst have been developed for the few known examples of N-methylation of amines with formic acid as C1 resource. The first example was reported in 2014. The employment of Pt/dppp [dppp = bis-(phenylphoshino) propane] led to catalytic methylation of amines using phenylsilane as a reductant [17]. Later on, a further example with Ru/triphos as the catalyst and methanesulfonic acidas an additive was reported, in which an excess amount of formic acid was used as both C1 resource and reductant [18]. A heterogeneous catalytic system with Pt/C has been developed using phenylsilane as the reductant [19]. Besides catalysts using noble metals, copper acetate has also been shown as an effective catalyst with phenylsilane as the reductant [20]. To date, Fu and Shang et al. reported the only transition-metal-free protocol using B(C6F5)3 as the catalyst and PMHS as the reductant under air-free conditions [21]. PMHS is a waste product of silicon industry [22]. Compared to other reductants [23-25], it is a cheap, non-toxic, and aerobically stable reductant suitable for large-scale production [26, 27]. Therefore, it is of practical significance to achieve the methylation reaction using PMHS instead of phenyl silane.
Herein, we report that simple inorganic salt, K2HPO4, can also catalyse the methylation of a range of amines using formic acid as the C1 source and hydrosilanes, especially PMHS, as the reductant under mild air-tolerant reaction conditions. Mechanistic studies suggest that the reaction occur through an acetal intermediate rather an amide intermediate.
Our study commenced with the methylation of N-methylaniline with formic acid as C1 source. Nine different hydrosilanes were screened for the reaction initially with a 10 mol% of K3PO4 as the catalyst and a 20 mol% of 18-crown-6 as the additive (Table 1, entries 1–9). Most of the silanes led to the desired product. Higher yields were observed for silanes with less bulkiness or hydrosiloxanes. Encouragingly, PMHS is one of the best among them (entry 8). Next, a few different inorganic salts were further screen to improve the yield for the reaction using PMHS as the reductant (entries 10–18). The yields vary significantly with the basicity of the salts. To our delight, a 60% yield was achieved with a moderate base, K2HPO4, as the catalyst (entry 12), suggesting that it should be important to have the just-right basicity to activate the hydrosilane. Various reaction conditions, including the loading of the catalyst, reaction temperature, reaction time and the solvent (Tables S1-S4 in Supporting information), were also screened. The optimized conditions were identified as below: 4.6 equiv. of formic acid, 4 equiv. of PMHS, 10 mol% K2HPO4, 20 mol% 18-crown-6, 4 Å molecular sieves as an additive, THF (2 mL) as the solvent, 80 ℃ for 12 h.
|
|
Table 1 Catalytic N-methylation of N-methylaniline using formic acid.a |
With the optimized conditions in hand, we next explored the scope of secondary amines for the reaction (Table 2). In general, good yields were obtained for aniline derivatives with various N-substituted alkyl groups(entries 1–5). In particular, the good tolerance of isopropyl and cyclohexyl indicates that the present protocol has a moderate tolerance of steric hindrance. Encouragingly, several synthetically useful functional groups, including olefinyl, chloro, bromo, nitro and cyano, were also tolerated by the present method (entries 6, 8–13). Unfortunately, for substrates with a strong electron-donating group, such as methoxy, N-formylated product was observed instead of the desired methylated one (Fig. S1 in Supporting information), suggesting that the present protocol is sensitive to the basicity of the amine. The significantly lower yield of diphenylaminerelative to N-benzyl aniline further suggests that such as basicity should not too weak. As such, only the N-formylated product was observed for aliphatic amines (Fig. S2 in Supporting information) It should be noted that N-formylated amines are desirable synthetic targets [28-30].
|
|
Table 2 N-Methylation of various amines with formic acid to form tertiary amines.a |
To test the scalability of the present methodology, a gram-scale reaction was also carried out for N-methylaniline (Scheme 1). The obtained isolated yield is close to that of its smaller-scale counterpart.

|
Download:
|
| Scheme 1. A gram-scale reaction of N-methylaniline. | |
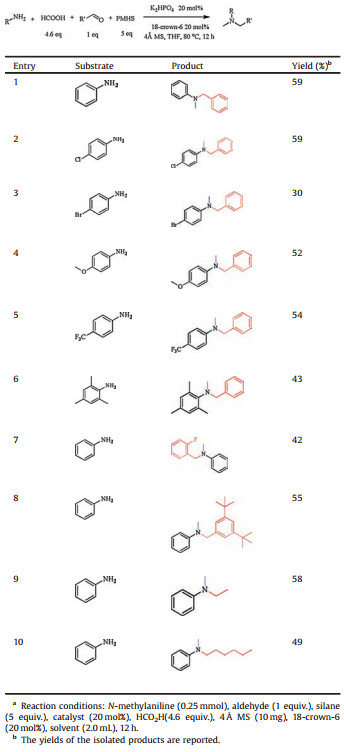
All our efforts to directly methylate primary amines with present protocol failed. An in situ reductive amination with aldehyde followed by trifluoroethylation with trifluoroacetic acid leading to dialkylation of primary amines was reported recently [31]. We applied the same strategy for primary amines and successful achieved a 59% yield for the desired product using aniline and benzaldehyde as model substrates (Table 3, entry 1). Due to the limited commercial availability of primary aniline derivatives, the scope of the present protocol was only probed preliminarily. Under the assistance of benzaldehyde, it was found that moderate yields can be obtained in general for substrates with para-chloro, bromo, methoxy and trifluoromethyl groups, showing a better tolerance of electronic properties than that for the direct N-methylation of secondary amines (vide supra). Encouragingly, trimethylaniline is also a suitable substrate (entry 6), indicating that the present protocol is fairlly sterically tolerant. Further experiments prove that the assisting aldehyde can be expanded to substrates other than benzaldehyde. Two examples, including 2-fluorobenzaldehyde (entry 7) and 3, 5-bis (tert-butyl) benzaldehyde (entry 8), were demonstrated. Unfortunately, the present protocol is not yet applicable to primary aliphatic amines due to the formation of tertiary amines via fast dialkylation with the aldehyde. But by reacting aliphatic aldehyde with aniline, the target product can be obtained (entries 9 and 10). This allows us to prepare methylationproducts containing straight chains.
|
|
Table 3 Indirect N-methylation of primary amines using formic acid and aldehyde.a |
Next, we studied the potential reaction mechanism for the present catalytic system. Two possible pathways can be envisioned (Scheme 2). In pathway (B), dehydrogenative coupling between formic acid and hydrosilane occurs first to afford silyl formate. Reduction of the formate can lead to the corresponding acetal, which can react with amine for C–N bond formation. Subsequent reduction generates the final product. In pathway (B), dehydrative condensation between formic acid and amine occurs first to form the corresponding formamide. Tandem reductions of the amide and the corresponding imine then afford the final product. Whether C–N bond formation takes place before the reduction or not is the key difference among the mechanisms. Since no reaction was observed for N, N'-formyl, methylaniline under the present optimized catalytic conditions (Eq. 1), pathway (B) is clearly ruled out. We next carried out a reaction between formic acid and PMHS in the absence of N-methylaniline, which led to the formation of a mixture of silyl formate and acetal as expected for pathway (A). The addition of N-methylaniline to such a mixture resulted in the disappearance of the acetal and the formation of the product (Figs. S3-S5 in Supporting information). As such, path (A) is the preferred reaction mechanism for the present catalytic system.

|
Download:
|
| Scheme 2. Proposed mechanisms for the K2HPO4-catalytic N-methylation. | |
In conclusion, we have developed a transition-metal-free, air-tolerant protocol for reductive N-methylation of amines using formic acid as the methylation agent and PMHS as the reduct-ant via simple inorganic base catalysis. Both the catalyst and the reductant are cheap and easily separable from the crude reaction product mixture, providing a convenient new tool for syntheses of N-methylated amines.
AcknowledgmentWe acknowledged the financial support by the National Natural Science Foundation of China (No. U1532135).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.064.
| [1] |
H.J. Arpe, S. Hawkins in Industrial Organic Chemistry, Wiley-VCH, 1997, pp. 3-5.
|
| [2] |
Amino group chemistry, in: A. Ricci (Ed.), From Synthesis to the Life Science, Wiley-VCH, Weinheim, 2007, pp. 2-10.
|
| [3] |
S.C. Holm, A.F. Siegle, C. Loosetal, Synthesis 40 (2010) 2278-2286. |
| [4] |
J.W. Lin, X.D. Li, Chin. Chem. Lett. 29 (2018) 1051-1057. DOI:10.1016/j.cclet.2018.05.017 |
| [5] |
D.A. Chiappetta, C. Alvarez-Lorenzo, A. Rey-Ricoetal, Eur. J. Pharm. Biopharm. 76 (2010) 24-37. DOI:10.1016/j.ejpb.2010.05.007 |
| [6] |
M. Stodulski, J. Mlynarski, Tetrahedron: Asymmetry 19 (2008) 970-975. DOI:10.1016/j.tetasy.2008.03.025 |
| [7] |
H.J. Lyu, H.L. Hu, J.Y. Rui, et al., Chin. Chem. Lett. 28 (2017) 482-486. DOI:10.1016/j.cclet.2016.10.025 |
| [8] |
W. Leitner, Angew. Chem. Int. Ed. 34 (1995) 2207-2221. DOI:10.1002/anie.199522071 |
| [9] |
Y. Du, Y.B. Shen, Y.L. Zhan, et al., Chin. Chem. Lett. 28 (2017) 1746-1750. DOI:10.1016/j.cclet.2017.05.018 |
| [10] |
K.J. Liu, X.L. Zeng, Y. Zhang, et al., Synthesis 50 (2018) 4637-4644. DOI:10.1055/s-0037-1610231 |
| [11] |
R. Fornika, H. Gorls, B. Seemann, W. Leitner, J. Chem. Soc. Chem Commun. (1995) 1479-1481. |
| [12] |
C. Ziebart, C. Federsel, M. Beller, et al., J. Am. Chem. Soc. 134 (2012) 20701-20704. DOI:10.1021/ja307924a |
| [13] |
K. Motokura, D. Kashiwame, A. Miyaji, T. Baba, Org. Lett. 14 (2012) 2642-2645. DOI:10.1021/ol301034j |
| [14] |
H.R. Jhong, S. Ma, P.J.A. Kenis, Curr. Opin. Chem. Eng. 2 (2013) 191-199. DOI:10.1016/j.coche.2013.03.005 |
| [15] |
Y.H. Li, X.J. Cui, K.W. Dong, K. Junge, M. Beller, ACS Catal. 7 (2012) 1077-1086. |
| [16] |
C. Fang, C.L. Lu, M.H. Liu, et al., ACS Catal. 6 (2016) 7876-7881. DOI:10.1021/acscatal.6b01856 |
| [17] |
I. Sorribes, K. Junge, M. BelIer, Chem. -Eur. J. 20 (2014) 7878-7883. DOI:10.1002/chem.201402124 |
| [18] |
S. Savourey, G. Lefevre, J.C. Berthet, T. Cantat, Chem. Commun. 50 (2014) 14033-14036. DOI:10.1039/C4CC05908E |
| [19] |
L. Zhu, L.S. Wang, B.J. Li, W. Li, B.Q. Fu. Catal. Sci. Technol. 6 (2016) 6172-6176. DOI:10.1039/C6CY00674D |
| [20] |
C. Qiao, X.F. Liu, X.L.L.N. He, Org. Lett. 19 (2017) 1490-1493. DOI:10.1021/acs.orglett.7b00551 |
| [21] |
M.C. Fu, R. Shang, W.M. Cheng, Y. Fu, Angew. Chem. Int. Ed. 54 (2015) 9042-9406. DOI:10.1002/anie.201503879 |
| [22] |
O. Jacquet, C.D.N. Gomes, M. Ephritikhine, T. Cantat, J. Am. Chem. Soc. 13 (2012) 2934-2937. |
| [23] |
M. Tao, F. Wu, T. Li, et al., Chin. Chem. Lett. 28 (2017) 97-100. DOI:10.1016/j.cclet.2016.05.028 |
| [24] |
L.H. Wang, D. Xiong, L.H. Jie, et al., Chin. Chem. Lett. 29 (2018) 907-910. DOI:10.1016/j.cclet.2018.05.007 |
| [25] |
T.T. Zhang, J.Y. Jiang, Y.H. Wang, Chin. Chem. Lett. 28 (2017) 307-311. DOI:10.1016/j.cclet.2016.07.029 |
| [26] |
D. Addis, S. Das, K. Junge, M. Beller, Angew. Chem. Int. Ed. 50 (2011) 6004-6011. DOI:10.1002/anie.201100145 |
| [27] |
B.H. Lipshutz, J.M. Servesko, Angew. Chem. Int. Ed. 42 (2003) 4789-4792. DOI:10.1002/anie.200352313 |
| [28] |
L.Y. Xie, S. Peng, L.H. Lu, et al., ACS Sustainable Chem. Eng. 6 (2018) 7989-7994. DOI:10.1021/acssuschemeng.8b01358 |
| [29] |
L.Y. Xie, S. Peng, F. Liu, et al., Adv. Synth. Catal. 360 (2018) 4259-4264. DOI:10.1002/adsc.201800918 |
| [30] |
W. Zheng, Y. Liu, H.L. Liu, et al., Chin. J. Org. Chem. 38 (2018) 2639-2647. DOI:10.6023/cjoc201805033 |
| [31] |
K.G. Andrews, R. Faizova, R.M. Denton, Nature Commun. 8 (2017) 15913. DOI:10.1038/ncomms15913 |
 2020, Vol. 31
2020, Vol. 31