b Qinghai Provincial Key Laboratory of Tibetan Medicine Research and Key Laboratory of Tibetan Medicine Research, Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Qinghai 810008, China;
c School of Chemistry and food Engineering, Changsha University of Science and Technology, Changsha 410114, China;
d School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China
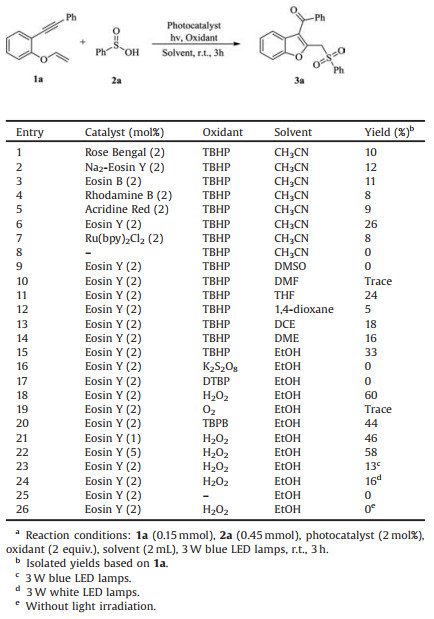
The cyclization of 1, n-enynes has emerged as a fascinating and powerful approach for the rapid assembly of various valuable complex ring systems due to its atom- and step-economic features [1, 2]. Through this methodology, various heterocycles including pyrroles, furans, pyrans, indoles, pyridines, and quinolines could be efficiently constructed [1, 2]. Benzofurans are privileged structures in many natural products and biologically active compounds [3]. Nevertheless, only few examples has been reported for the synthesis of functionalized benzofurans using the 1, 6-enyne cyclization strategy [4].
Sulfone groups are of great importance in term of their wide applications in organic synthesis, materials, medicinal chemistry [5]. Consequently, numerous efforts have been devoted to the construction of sulfone-containing compounds [6]. In 2017, Wu and Jiang reported a AgNO3 catalyzed oxidative cyclization reactions of 1, 6-enynes and sodium sulfinates leading to sulfonylated benzofurans in the presence of 2 equiv. of K2S2O8 (Scheme 1a) [7]. In 2018, Sun and Liu described NH4I mediated a radical cascade cyclization of 1, 6-enynes with DMSO for the synthesis of methylsulfonylated benzofurans (Scheme 1b) [8]. However, both well developed reactions require the use of transition metal catalyst or higher reaction temperature, which might limit their practical applications in the synthetic chemistry. Therefore, the development of new, mild, and metal-free method is still desirable.
|
Download:
|
| Scheme 1. Methods for synthesis of sulfonylated benzofurans. | |
Recently, visible-light catalysis as a powerful synthetic protocol has attracted great interests of chemists to synthesize various functionalized organic molecules owing to its mild, clean, and renewable advantages [9]. As our ongoing interest in photochemical reactions [10] and the construction of sulfone-containing compounds [11], herein, we wish to report a new visible-light-induced method for the synthesis of sulfonylated benzofurans via Eosin Y catalyzed oxidative cyclization reaction of 1, 6-enynes and arylsulfinic acids (Scheme 1c). The present protocol provides a convenient and metal-free approach to access various sulfonylated benzofurans from simple starting materials under mild conditions, in which the C–S, C-C and C=O bonds could be sequentially formed in one pot procedure.
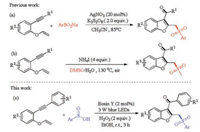
Initially, 1-(phenylethynyl)-2-(vinyloxy)benzene (1a) and benzenesulfinic acid (2a) were chosen as model substrates to optimize the reaction conditions under the irradiation of 3 W blue LED lamps at room temperature. The product 3a was isolated in 10% yield when the model reaction was carried out in CH3CN by using of Rose Bengal (2 mol%) and TBHP (70% solution in water, 2 equiv.) as the photocatalyst and oxidant, respectively (Table 1, entry 1). Next, a series of photocatalysts were screened to improve the reaction efficiency (entries 2–7). To our delight, the yield of product 3a was increased to 26% when Eosin Y was employed as catalyst (entry 6). No transformation was observed in the absence of photocatalyst (entry 8). Furthermore, various solvents were investigated and EtOH was found to be the optimal reaction medium (entry 15). Subsequently, the screening of other oxidants found that the best yield (60%) was obtained when H2O2 was used as the oxidant (entry 18). The decrease of catalyst loading would lead to the lower reaction efficiency (entry 21). Moreover, when the reaction was carried out under irradiation with 3 W green or 3 W white LED lamps, the corresponding product 3a was obtained in only 13% and 16% yields (entries 23 and 24). In addition, no product was detected when the reaction was performed in the absence of oxidant or visible-light irradiation (entries 25 and 26).
|
|
Table 1 Screening of the reaction conditions.a |
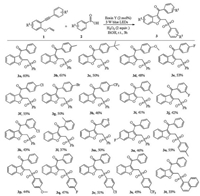
With the optimal conditions in hand, the scope of a variety of 1, 6-enyne derivatives was firstly examined (Scheme 2). In general, a range of 1, 6-enyne derivatives containing para-substituted electron-donating or electron-withdrawing groups of the phenyl ring were tolerated in this reaction process leading to corresponding products 3b-3h in moderate to good yields. It should be noted that halogen substituents including F, Cl, and Br were all suitable for this reaction, which made this method useful for further transformations. The steric hindrance of substituents on the phenyl ring had a great effect on the reaction efficiency, and the corresponding products 3i-3l were obtained in relatively lower yields. Notably, heterocyclic substituted 1, 6-enyne was also compatible for the reaction to afford the corresponding 3m in 50% yield. Next, the scope of different arylsulfinic acids was investigated. Arylsulfinic acids bearing electron-donating or electron-withdrawing groups were all concerted to the corresponding compounds 3n-3s in moderate yields. It was found that naphthalene-2-sulfinic acid could also be used in this reaction, but leading to the desired product 3t in a relatively lower yield. Unfortunately, none of the desired product was detected when alkyl sulfinic acid such as methanesulfinic acid was investigated in the present reaction system.
|
Download:
|
| Scheme 2. Substrate scopes. Reaction condition: 1 (0.15 mmol), 2 (0.45 mmol), Eosin Y (2 mol%), H2O2 (2 equiv), EtOH (2 mL), 3 W blue LED lamps, r.t., 3 h. Isolated yields based on 1. | |
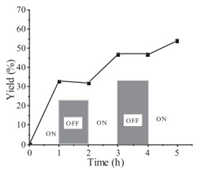
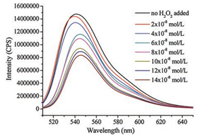
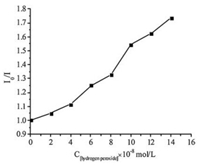
A series of control experiments were carried out to get insights into the reaction mechanism. Initially, none of the desired product 3a was obtained when radical scavenger 2, 2, 6, 6-tetramethyl-1-piperidinyloxy (TEMPO) was added in the model reaction, suggesting that this transformation might involve a radical process (Scheme 3a). Furthermore, when H2O18 (3 equiv.) was added in the model reaction under standard conditions (Scheme 3b), the O18-3a was mainly obtained. This result demonstrated that the carbonyl oxygen atom came from water. In addition, the on/off light-illumination experiments were also performed, and the result indicated that the continuous light illumination is essential for this transformation (Fig. 1). Finally, to elucidate an energy transfer process between photocatalyst and H2O2, fluorescence quenching (Stern–Volmer) experiments were also conducted. The emission intensity of the excited Eosin Y was dramatically decreased along with the increasing of the concentration of H2O2 (Fig. 2, Fig. 3). These results indicated that an electron transfer process occurred between Eosin Y and H2O2 under the standard reaction conditions.

|
Download:
|
| Scheme 3. Control experiments. | |
|
Download:
|
| Fig. 1. Visible light irradiation on/off experiment. | |
|
Download:
|
| Fig. 2. Quenching of Eosin Y fluorescence emission in the presence of H2O2. | |
|
Download:
|
| Fig. 3. Stern-volmer plots. | |
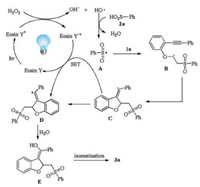
Based on the above experimental results and previous reports [4, 7, 8, 12, 13], we proposed a possible reaction pathway as shown in Scheme 4. Initially, the excited state Eosin Y* was generated from Eosin Y under visible-light irradiation [12]. Then, a single-electron transfer (SET) from the excited state of Eosin Y* to H2O2 gave Eosin Y•+ and hydroxide radical. Subsequently, the abstraction of a hydrogen from benzenesulfinic acid 2a by hydroxide radical afforded sulfonyl radical A. Next, the addition of sulfonyl radical A to a vinyl moiety of 1a to form the alkyl radical intermediate B. Next, intramolecular cyclization of alkyl radical with an alkyne moiety gave vinyl radical intermediate C, which is further oxidized by Eosin Y•+ to form the carbon cation intermediate D through a SET process. The interaction of carbon cation intermediate D with H2O would produce the enolate adduct E. Finally, the desired product 3a was produced by the isomerization of intermediate E.
|
Download:
|
| Scheme 4. Possible reaction pathway. | |
In summary, we have developed a convenient and metal-free protocol for the construction of various sulfonylated benzofurans via visible-light-promoted oxidative cyclization reaction of 1, 6-enynes and arylsulfinic acids. This reaction could be achieved in one pot procedure via sequential C–S, C-C and C=O bond formation. The present new strategy offers a mild and green approach to access sulfonylated benzofurans from simple and readily available materials, which may have potential application in synthetic chemistry. Further synthetic application and mechanistic investigation is ongoing in our lab.
AcknowledgmentsThis work was supported by the Natural Science Foundation of Shandong Province (No. ZR2018MB009), the International Cooperation Project of Qinghai Province (No. 2018-HZ-806), the Qinghai Key Laboratory of Tibetan Medicine Research (No. 2017-ZJ-Y11), and the National Natural Science Foundation of China (No. 21302109).
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.05.041.
| [1] |
(a) J. Qiu, B. Jiang, Y. Zhu, et al., J. Am. Chem. Soc. 137 (2015) 8928-8931; (b) A. Wang, Y. Zhu, S. Wang, et al., J. Org. Chem. 81 (2016) 1099-1105; (c) Y. Zhu, B. Jiang, W. Hao, et al., Chem. Commun. 52 (2016) 1907-1910; (d) P. Zhou, J. Wang, T. Zhang, et al., Chem. Commun. 54 (2018) 164-167; (e) H. Sha, F. Liu, J. Lu, et al., Green Chem. 20 (2018) 3476-3485; (f) J. Qi, Q. Teng, N. Thirupathi, C. Tung, Z. Xu, Org. Lett. 21 (2019) 692-695; (g) Y. Yu, Z. Cai, W. Yuan, P. Liu, P. Sun, J. Org. Chem. 82 (2017) 8148-8156. |
| [2] |
(a) J. Li, W. Zhang, X. Wei, et al., J. Org. Chem. 82 (2017) 6621-6628; (b) Q. Deng, L. Tan, Y. Xu, P. Liu, P. Sun, J. Org. Chem. 83 (2018) 6151-6161; (c) H. Li, W. Hao, M. Wang, et al., Org. Lett. 20 (2018) 4362-4366; (d) X. Li, S.Zhuang, X. Fang, P. Liu, P. Sun, Org. Biomol. Chem. 15 (2017) 1821-1827; (e) C. Zhang, H. Jiang, S. Zhu, Chem. Commun. 53 (2017) 2677-2680; (f) H. Sha, T. Xu, F. Liu, et al., Chem. Commun. 54 (2018) 10415-10418. |
| [3] |
(a) B.S. Yun, H.C. Kang, H. Koshino, S.H. Yu, I.D. Yoo, J. Nat. Prod. 64 (2001) 1230-1231; (b) Y. Liu, M. Kubo, Y. Fukuyama, J. Nat. Prod. 75 (2012) 2152-2157; (c) J.S. Li, P.P. Yang, G.Q. Chen, et al., Asian J. Org. Chem. 8 (2019) 246-250; (d) J.S. Li, Q. Yang, G.Q. Chen, Z.W. Li, P.M. Huang, ChemistrySelect 3 (2018) 10621-10623; (e) Y. Liu, L. Chen, Z. Wang, et al., J. Org. Chem. 84 (2019) 204-215; (f) J.S. Li, D.M. Fu, Y. Xue, et al., Tetrahedron 71 (2015) 2748-2752; (g) J.S. Li, F.F. Cai, Z.W. Li, et al., RSC Adv. 4 (2014) 474-478; (h) Y. You, K. Zhou, B. Guo, et al., ACS Sens. 4 (2019) 774-779; (i) Q. Liang, Y. Zhang, M. Zeng, et al., Analyst 143 (2018) 2411-2415. |
| [4] |
(a) S. Jana, A. Verma, R. Kadu, S. Kumar, Chem. Sci. 8 (2017) 6633-6644; (b) X. Xia, W. He, G. Zhang, D. Wang, Org. Chem. Front. 6 (2019) 342-346; (c) N. Sakiyama, K. Noguchi, K. Tanaka, Angew. Chem. Int. Ed. 51 (2012) 5976-5980; (d) M. Hu, B. Liu, X. Ouyang, R. Song, J. Li, Adv. Synth. Catal. 357 (2015) 3332-3340. |
| [5] |
(a) M.D. McReynolds, J.M. Dougherty, P.R. Hanson, Chem. Rev. 104 (2004) 2239-2258; (b) R.P. Baumann, K. Ishiguro, P.G. Penketh, et al., Biochem. Pharmacol. 81 (2011) 1201-1210; (c) Q. Liang, Y. Zhang, M. Zeng, et al., Toxicol. Res. 7 (2018) 521-528; (d) W. Li, G. Yin, L. Huang, et al., Green Chem. 18 (2016) 4879-4883; (e) K. Sun, X. Wang, F. Fu, et al., Green Chem. 19 (2017) 1490-1493; (f) K. Sun, Z. Shi, Z. Liu, et al., Org. Lett. 20 (2018) 6687-6690; (g) J.S. Li, Y. Xue, Z.W. Li, et al., Synlett 24 (2013) 2003-2005. |
| [6] |
(a) K. Sun, X.L. Chen, S.J. Li, et al., J. Org. Chem. 83 (2018) 14419-14430; (b) C. Wu, P. Yang, Z. Fu, et al., J. Org. Chem. 81 (2016) 10664-10671; (c) L.Y. Xie, Y.J. Li, J. Qu, et al., Green Chem. 19 (2017) 5642-5646; (d) L.Y. Xie, S. Peng, F. Liu, et al., Org. Chem. Front. 5 (2018) 2604-2609; (e) L.Y.Xie, S.Peng, J.X.Tan, etal., ACS Sustainable Chem. Eng. 6 (2018)16976-16981. |
| [7] |
W. Wu, S. Yi, W. Huang, D. Luo, H. Jiang, Org. Lett. 19 (2017) 2825-2828. DOI:10.1021/acs.orglett.7b00980 |
| [8] |
J. Zhang, S. Cheng, Z. Cai, P. Liu, P. Sun, J. Org. Chem. 83 (2018) 9344-9352. DOI:10.1021/acs.joc.8b01265 |
| [9] |
(a) W. Liu, X. Yang, Y. Gao, C.J. Li, J. Am. Chem. Soc. 139 (2017) 8621-8627; (b) X. Li, X. Fang, S. Zhuang, P. Liu, P. Sun, Org. Lett. 19 (2017) 3580-3583; (c) H. Wang, Y. Li, Z. Tang, et al., ACS Catal. 8 (2018) 10599-10605; (d) S. Liu, J. Jie, J. Yu, X. Yang, Adv. Syn. Catal. 360 (2018) 267-271; (e) S. Liu, W. Pan, S. Wu, et al., Green Chem. 21 (2019) 2905-2910; (f) D. Liu, W. Ding, Q. Zhou, et al., Org. Lett. 20 (2018) 7278-7282; (g) T.Y. Shang, L.H. Lu, Z. Cao, et al., Chem. Commun. 55 (2019) 5408-5419; (h) R. Li, X. Chen, S. Wei, et al., Adv. Synth. Catal. 360 (2018) 4807-4813. |
| [10] |
(a) W. Wei, P. Bao, H. Yue, et al., Org. Lett. 20 (2018) 5291-5295; (b) W. Wei, L. Wang, P. Bao, et al., Org. Lett. 20 (2018) 7125-7130; (c) H. Cui, W. Wei, D. Yang, et al., Green Chem. 19 (2017) 3520-3524; (d) W. Wei, H. Cui, H. Yue, D. Yang, Green Chem. 20 (2018) 3197-3202; (e) L. Wang, P. Bao, W. Liu, et al., Chin. J. Org. Chem. 38 (2018) 3189-3196; (f) Q. Liu, L. Wang, H. Yue, et al., Green Chem. 21 (2019) 1609-1613. |
| [11] |
(a) J. Wen, W. Wei, S. Xue, et al., J. Org. Chem. 80 (2015) 4966-4972; (b) L. Wang, H. Yue, D. Yang, et al., J. Org. Chem. 82 (2017) 6857-6864; (c) W. Wei, C. Liu, D. Yang, et al., Chem. Commun. 49 (2013) 10239-10241; (d) W. Wei, J. Li, D. Yang, et al., Org. Biomol. Chem. 12 (2014) 1861-1864; (e) W. Wei, J. Wen, D. Yang, et al., Chem. Commun. 51 (2015) 768-771; (f) W. Wei, J. Wen, D. Yang, et al., Green Chem. 16 (2014) 2988-2991; (g) W. Wei, J. Wen, D. Yang, et al., Org. Biomol. Chem. 12 (2014) 7678-7681; (h) W. Wei, J. Wen, D. Yang, et al., RSC Adv. 5 (2015) 4416-4419; (i) W. Wei, H. Cui, D. Yang, et al., Org. Chem. Front. 4 (2017) 26-30; (j) P. Bao, L. Wang, Q. Liu, et al., Tetrahedron Lett. 60 (2019) 214-218. |
| [12] |
(a) W. Yang, S. Yang, P. Lia, L. Wang, Chem. Commun. 51 (2015) 7520-7523; (b) W. Wei, H. Cui, D. Yang, et al., Green Chem. 19 (2017) 5608-5613; (c) G. Zhang, L. Zhang, H. Yi, et al., Chem. Commun. 52 (2016) 10407-10410; (d) W.Wei, L.Wang, H.Yue, etal., ACSS ustainable Chem. Eng. 6 (2018)17252-17257; (e) Y.Y. Liu, X.Y. Yu, J.R. Chen, et al., Angew. Chem. Int. Ed. 56 (2017) 9527-9531. |
| [13] |
(a) D. Xia, T. Miao, P. Li, L. Wang, Chem. -Asian J. 10 (2015) 1919-1925; (b) Y. Li, F. Ma, P. Li, T. Miao, L. Wang, Adv. Synth. Catal. 361 (2019) 1606-1616; (c) M. Huang, Y. Zhu, W. Hao, et al., Adv. Synth. Catal. 359 (2017) 2229-2234; (d) V. Srivastava, P.P. Singh, RSC Adv. 7 (2017) 31377-31392; (e) P. Qian, Y. Deng, H. Mei, et al., Org. Lett. 19 (2017) 4798-4801. |
 2020, Vol. 31
2020, Vol. 31