b Key Laboratory of Advanced Display and System Applications, Ministry of Education, Shanghai University, Shanghai 200072, China
Since efficient organic light-emitting diodes (OLEDs) were reported by Tang and Van Slyke [1], OLEDs have attracted more and more attention due to their potential applications in flat panel displays [2-6]. Although numerous classes of light emitting organic semiconductors have been designed and developed, deep-blue emitters with high efficiency are still scarce and efforts are still continuing in order to find efficient pure blue light emitting materials for OLEDs [7-12]. Blue light emitting materials play important roles in energy-efficient solid-state lighting and smart display devices. Energy-efficient OLEDs are considered one of the most competitive candidates for next generation smart displays and particularly for future energy-saving lighting sources [13, 14]. Recently, much effort has been devoted to attempts to generate white OLEDs comprising both fluorescent and phosphorescent materials [15, 16]. Efficient blue-emitting materials are also one of the important key elements for fabricating white OLED devices [17-21]. Among various blue light-emitting materials, fluorene-based polymers or oligomers have attracted extensive interest due to their excellent blue light-emitting ability, easily processing, good thermal and electrochemical stabilities [22-28]. Although many blue light-emitting materials based on fluorenes have been reported, the EL efficiencies of these deep-blue OLEDs (Commission Internationale de L'Eclairage (CIE) y coordinate value < 0.15 along with an (x+y) value < 0.30) are rather poor compared to those of sky-blue OLEDs (CIE coordinates of x=0.15 and y > 0.15) [7]. Therefore, the search for new efficient deep-blue fluorescent materials with high performance remains a major challenge.
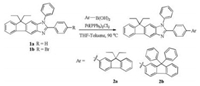
In the search of new luminophores for use in light-emitting materials, we have successfully synthesized a series of new fluorene derivatives based on 3, 9-dihydro-9, 9-dioctylfluoreno[3, 2-d]imidazole (DOFI), 10, 10-dioctyl-3, 6-dihydrofluoreno[2, 3-d:6, 7-d']diimidazoles (DOFDIs) [29, 30] and 9, 9-diethyl-1, 2-diaryl-1, 9-dihydrofluoreno[2, 3-d]imidazoles [30], which emit blue fluorescence and have acceptable fluorescence quantum yields. In this letter, we report the synthesis and blue light-emitting properties of two new 9, 9-diethyl-1, 2-diaryl-1, 9-dihydrofluoreno[2, 3-d]imidazoles (2a, 2b) by introducing a fluorene group into the 9, 9-diethyl-1, 2-diphenyl-1, 9-dihydrofluoreno[2, 3-d]imidazole (1a) core. The synthesis and characterization of 2a-b, including their photophysical and electrochemical properties, thermal stability property, and electroluminescence (EL) performance of 2b were well performed. Compounds 2a-b were synthesized by palladium-catalyzed Suzuki cross-coupling reaction of 2-(4-bromophenyl)-9, 9-diethyl-1-phenyl-1, 9-dihydrofluoreno[2, 3-d]imidazole (1b) [31] and fluorene boric acid as outlined in Scheme 1. Fig. 1 and Table 1 display the absorption and photoluminescence (PL) spectra upon excitation at each excitation wavelength (λex) at room temperature in CH2Cl2 and DMSO solutions. As shown in Fig. 1, both compounds 2a-b display almost the same absorption characteristics, showing two peaks at ca. 280, 351 nm and 289, 352 nm, respectively, which can be attributed to different π–π* transitions. The UV–vis absorption maxima of compounds 2a-b were red-shifted by ca. 14 nm relative to that of 1a, due to the elongation of conjugation length. It was observed that the absorption spectra changed very little with the increase of solvent polarity although there was a tendency of a longer λmax in DMSO, indicating that there was no charge transfer in the ground state. With the introduction of fluorene group, the peaks of the fluorescent spectra shift from the near UV region to the blue region. In CH2Cl2 solution, compound 2a showed emission peak at 428 nm (Fig. 1a) and the fluorescence quantum yield is 0.86, which is higher than that of quinine sulfate (ΦFL = 0.55). It is interesting to note that compound 2b shows strong blue fluorescence-emitting ability with ΦFL value of 0.55, with PL emission peak at 430 nm slightly red-shifted by 2 nm relative to 2a. Moreover, both compounds were slightly red-shifted in DMSO relative to those in CH2Cl2. Comparing the absorption and fluorescence spectra of 2a, 2b and 1a indicates that absorption and fluorescence are determined by the whole conjugation system and substitutions at the C-9 position of the fluorene have little influence on the optical properties of the molecule.
|
Download:
|
| Scheme 1. The synthetic route of the compounds. | |

|
Download:
|
| Fig. 1. UV–vis spectra (a, 5 × 10-5 mol/L) and fluorescence spectra (b, 5 × 10-7 mol/L) of compounds in CH2Cl2; (c) EL spectrum of compound 2b; (d) The J-V-L characteristics; (e) The ηc-L–ηp characteristics; (f) the EQE%-L. | |
|
|
Table 1 The absorption characteristics and optical properties of the three compounds. |
The HOMO and LUMO of 2a and b were determined by the cyclic voltammetrical analysis and UV–vis absorption edge. The measured HOMO levels for 2a and 2b are −5.56 and −5.53 and their LUMO levels are −2.38 and −2.42, respectively. Therefore, substitutions at the C9 position have little impact on their frontier energy levels. Apparently, these two compounds exhibit wide bandgaps and this is suitable for their use as blue emitters. To provide a better insight into the geometries and electron-state-density distributions of the fluorophores, DFT calculation at B3LYP/6-31G(d) level was performed, as provided in Fig. 2. In the optimized ground state geometry, 2a and 2b show twisted structures with a dihedral angle around 15° between the phenyl spacer and plane of fluoreno[2, 3-d]imidazole. The electron cloud distribution of the two compounds is almost similar. The HOMO state density of the two fluorophores mainly distributes on the fluorenoimidazole skeleton and the bridging phenyl ring, spreading on the phenyl ring of the terminal fluorene. On the other hand, the LUMO state electron density mainly locates on the benzoimidazole ring, N-1 phenyl group and the interconnected phenyl ring with a minor contribution from the phenyl ring of the fluorene unit. The calculated HOMO levels were −5.19, −5.42 eV, while the LUMO levels were −1.45, −1.76 eV for 2a and 2b, respectively. The predicted HOMO and LUMO energies show good agreement with the experimental results although there are some discrepancies.

|
Download:
|
| Fig. 2. Optimal structures and the distributions of the frontier levels (HOMO, LUMO) for 2a and b. | |
Compound 2b exhibited good thermal stability with a high decomposition temperature (Td: corresponding to 5 wt% mass loss) of 350 ℃ and a high glass transition temperature (Tg) of 490 ℃, which is suffice for application in OLEDs. To explore the potential of 2b as active ingredient, we fabricated blue fluorescence OLEDs by multi-layer vacuum deposition with the device configuration of ITO / HATCN (5 nm) / NPB (20 nm) / TCTA (10 nm) / CBP: 2b (5%) (20 nm) / TMPYPB (40 nm) / LiQ (1 nm) / Al (80 nm), in which ITO (indium tin oxide) was used as anode, HATCN (1, 4, 5, 8, 9, 11-hexaaza triphenylene-hexacarbonitrile) used as the hole injection layer (HIL), NPB (N, N'-bis-(1-naphthyl)-l)-N, N'-diphenyl-[1, 1′-biphenyl]-4, 4′-diamine) used as the hole blocking layer (HBL), TCTA (4, 4′, 4′'-tris(carbazol-9-yl)-triphenylamine) used as the exciton blocking layer (EBL), the 2b (5% concentration) doped in the CBP (4, 4′-N, N'-dicarbazole-biphenyl) host material was used as the emitting layer (EML), TmPYPB (3, 3′-(5′-(3-(pyridin-3-yl)phenyl)-[1, 1′:3′, 1′'-terphenyl]-3, 3′'-diyl)dipyridine) used as the electron transporting layer (ETL), LiQ (lithium 8-hydroxyquinolate) used as the electron injecting layer (EIL), Al was used as the cathode. The EL spectrum of this device shows blue emission maxima at around 428 nm, which was almost the same as compared to that obtained from CH2Cl2 solution. This doped device is located in the deep-blue region with CIE (x, y) values of (0.1590, 0.0465), exhibits a maximum luminance of 1272 cd/m2 at 4 V, a maximum luminance efficiency of 1.45 cd/A, a maximum power efficiency of 0.85 lm/W and a maximum external quantum efficiency of 4.72%.
In summary, two 2-(4-(9, 9-disubstitued-9H-fluoren-2-yl)phenyl)-9, 9-diethyl-1-phenyl-1, 9-dihydrofluoreno[2, 3-d]imidazole derivatives 2a and 2b were designed and synthesized for application in doped blue OLEDs. The fabricated device based on 2b doping into 4, 4′-N, N'-dicarbazole-biphenyl (5%) as an emitter shows a good performance with maximum EL peak at 428 nm, a maximum luminance of 1272 cd/m2 at 4 V, a maximum luminance efficiency of 1.45 cd/A, a maximum power efficiency of 0.85 lm/W and CIE coordinate (0.1590, 0.0465), approaching EBU deep blue standard [32]. Further research regarding the incorporation of these systems usable as organic light-emitting diodes will be reported in due course.
AcknowledgmentThis study was supported by the National Natural Science Foundation of China (Nos. 81202402, 21272154).
| [1] |
C.W. Tang, S.A. VanSlyke, Appl. Phys. Lett. 51 (1987) 913-915. DOI:10.1063/1.98799 |
| [2] |
Y.J. Luo, Z.Y. Lu, Y. Huang, et al., Chin. Chem. Lett. 27 (2016) 1223-1230. DOI:10.1016/j.cclet.2016.06.002 |
| [3] |
Z. Huang, S. Xiang, Q. Zhang, et al., J. Mater. Chem. C 6 (2018) 2379-2386. |
| [4] |
Z.H. Li, M.J. Xiong, M.S. Wong, Chin. Chem. Lett. 18 (2007) 823-826. DOI:10.1016/j.cclet.2007.05.016 |
| [5] |
H.X. Xu, F. Wang, K.X. Wang, et al., Dyes Pigm. 155 (2018) 84-92. DOI:10.1016/j.dyepig.2018.03.030 |
| [6] |
C.K. Liu, Y.H. Chen, Y.J. Long, et al., Dyes Pigm. 157 (2018) 101-108. DOI:10.1016/j.dyepig.2018.04.035 |
| [7] |
M. Zhu, C. Yang, Chem. Soc. Rev. 42 (2013) 4963-4976. DOI:10.1039/c3cs35440g |
| [8] |
P.K. Samanta, D. Kim, V. Coropceanu, et al., J. Am. Chem. Soc. 139 (2017) 4042-4051. DOI:10.1021/jacs.6b12124 |
| [9] |
Y. Tan, Z. Zhao, L. Shang, et al., J. Mater. Chem. C 5 (2017) 11901-11909. DOI:10.1039/C7TC04089J |
| [10] |
A. Abdurahman, A. Obolda, Q. Peng, et al., Dyes Pigm. 153 (2018) 10-17. DOI:10.1016/j.dyepig.2018.02.002 |
| [11] |
M. Jung, J. Lee, H. Jung, et al., Dyes Pigm. 158 (2018) 42-49. DOI:10.1016/j.dyepig.2018.05.024 |
| [12] |
T. Shan, Y. Liu, X. Tang, et al., ACS Appl. Mater. Inter. 8 (2016) 28771-28779. DOI:10.1021/acsami.6b10004 |
| [13] |
N. Thejokalyani, S.J. Dhoble, Renew. Sust. Energ. Rev. 32 (2014) 448-467. DOI:10.1016/j.rser.2014.01.013 |
| [14] |
J. Moon, E. Kim, S.K. Park, et al., Org. Electron. 26 (2015) 273-278. DOI:10.1016/j.orgel.2015.07.046 |
| [15] |
L.S. Cui, Y. Liu, X.Y. Liu, et al., ACS Appl. Mater. Inter. 7 (2015) 11007-11014. DOI:10.1021/acsami.5b02541 |
| [16] |
J. Wan, C.J. Zheng, M.K. Fung, et al., J. Mater. Chem. 22 (2012) 4502-4510. DOI:10.1039/c2jm14904d |
| [17] |
N. Sun, Q. Wang, Y. Zhao, et al., Adv. Mater. 26 (2014) 1617-1621. DOI:10.1002/adma.201304779 |
| [18] |
Y. Sun, N.C. Giebink, H. Kanno, et al., Nature 440 (2006) 908-912. DOI:10.1038/nature04645 |
| [19] |
G. Schwartz, K. Fehse, M. Pfeiffer, et al., Appl. Phys. Lett. 89 (2006) 083509-083512. DOI:10.1063/1.2338588 |
| [20] |
X. Yang, Z. Wang, S. Madakuni, et al., Adv. Mater. 20 (2008) 2405-2409. DOI:10.1002/adma.200702940 |
| [21] |
K.T. Kamtekar, A.P. Monkman, M.R. Bryce, Adv. Mater. 22 (2010) 572-582. DOI:10.1002/adma.200902148 |
| [22] |
W. Sun, N. Zhou, Y. Xiao, et al., Chem. Asian J. 12 (2017) 3069-3076. DOI:10.1002/asia.201701292 |
| [23] |
W. Sun, N. Zhou, Y. Xiao, et al., Dyes Pigm. 154 (2018) 30-37. DOI:10.1016/j.dyepig.2018.02.041 |
| [24] |
K.T. Wong, R.T. Chen, F.C. Fang, et al., Org. Lett. 7 (2005) 1979-1982. DOI:10.1021/ol050547o |
| [25] |
C.G. Zhen, Z.K. Chen, Q.D. Liu, et al., Adv. Mater. 21 (2009) 2425-2429. DOI:10.1002/adma.200900095 |
| [26] |
J.H. Jou, S. Kumar, P.H. Fang, et al., J. Mater. Chem. C 3 (2015) 2182-2194. DOI:10.1039/C4TC02547D |
| [27] |
C.C. Wu, Y.T. Lin, H.H. Chiang, et al., Appl. Phys. Lett. 81 (2002) 577-579. DOI:10.1063/1.1493669 |
| [28] |
N. Matsumoto, T. Miyazaki, M. Nishiyama, et al., J. Phys. Chem. C 113 (2009) 6261-6266. DOI:10.1021/jp809024h |
| [29] |
J.G. Guo, Y.M. Cui, H.X. Lin, et al., J. Photoch. Photobiol. 219 (2011) 42-49. DOI:10.1016/j.jphotochem.2011.01.014 |
| [30] |
H.F. Chen, Y.M. Cui, J.G. Guo, et al., Dyes Pigm. 94 (2012) 583-591. DOI:10.1016/j.dyepig.2012.03.004 |
| [31] |
T.Q. Wang, S.L. Zhao, W.M. Zhang, H.X. Lin, Y.M. Cui, Monatsh. Chem. 147 (2016) 1991-1999. DOI:10.1007/s00706-016-1716-8 |
| [32] |
E.J. Lee, Y.H. Ha, Ieee Trans. Consum. Electron. 44 (1998) 10-15. DOI:10.1109/30.663725 |
 2020, Vol. 31
2020, Vol. 31 

