b Department of Mechanical Engineering, California State University, Los Angeles, CA 90032, United States;
c Energy College, Beijing University of Chemical Technology, Beijing 100029, China
Smart self-cleaning surfaces have widely variety promising applications, e.g., in super-grip tires, reliable climbing robots, and manipulation/securing devices in and outside of space stations, as well as on solar panels [1–4]. These smart surfaces have the ability to actively remove contaminates and debris without relying on extra cleaning efforts and now classified into four different types: TiO2–based self-cleaning surfaces, Lotus effect based self-cleaning surfaces, antifouling self-cleaning surfaces inspired by underwater organisms and the gecko inspired self-cleaning surfaces [5]. Among them, gecko owns the remarkable dry self-cleaning systems. Gecko's hierarchical toe pad structure consists the lamellae (mesoscale), the setae (micrometer scale) and the spatulae (nanoscale). Simply with the van der Waal force and the digital hyperextension effect, gecko can dislodge up to 80% of contaminant particles within as few as 4 steps [6–12]. At first, dry contact self-cleaning was first thought to take place due to an energetic disequilibrium at the interfaces between the particle, the substrate, and the single spatula or spatula cluster. Particle rolling and sliding between the substrate and setae during detachment were proposed to affect the dry self-cleaning capability [13]. For example, Menguc and Sitti claimed that particle rolling between nearby fibers or lamellae grooves was a dominant mechanism for the dry self-cleaning [14]. They demonstrated that a specific size ratio between contaminants and microfiber tips was important for this mechanism to be effective. Hu et al. studied the gecko's digital hyperextension (DH) motion and confirmed it played a crucial role in the gecko's self-cleaning ability [15]. Although the self-cleaning mechanism can be partly revealed, it is still can not fully explained the mechanism behind the gecko's unique reversible adhesion system [16–24]. Besides, simple and low costly self-cleaning fabrication method is still waiting to be explored.
In this study, we develop a straight and low-cost method to fabricate dry self-cleaning surfaces. The self-cleaning surfaces showed up to 24.3% self-cleaning rate with as few as 4 steps. DFT Simulation revealed that the inner energy of the Fe3O4/PDMS system increased in the loading process, which could be favorable to the formation of non-joint-chain dinethylsiloxane structures and thus lead to the enhanced adhesion forces performances of the surfaces. This straight forward method provides a straightforward route of producing a robust dry self-cleaning surface with promising application in aerospace, biomedical and microfluidic fields, etc.
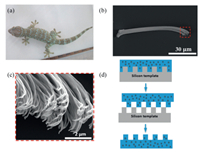
The structure hierarchy of the gecko adhesive system is in Figs. 1a–c. The biomimetic fibrillar surfaces were fabricated using photolithography defined silicon wafer as the 'negative' template. A mixture of the PDMS-magnetic nanoparticles was poured into it, cured, and followed by a peeling procedure to obtain the gecko-inspired composite surfaces. The protocol is schematically shown in Fig. 1d. In this part, we use AFM for the adhesion test. The tip-less AFM cantilever was modified with a SiO2 particle attached to it underneath as shown in Fig. 2a, which serving as the probe to eliminate the effect of misalignment during engagement. The AFM cantilever used in this experiment was 225 μm in length, 28 μm in width and 3 μm in thickness, with an average spring constant of 2.8 N/m and an average frequency of 75 kHz. Uniformity of the as-prepared composite adhesives was demonstrated in Fig. 2b, which provides a basis for studying the mechanical properties of single or a few pillars/fibrils of the samples using atomic force microscope (AFM) and the typical force curves as shown in Fig. 2c. The diameter of the probe was 5.6 μm, and the diameter of the fiber was ~22 μm (Fig. 2d). Since the base substrate of the sample was not a completely flat surface, we were unable to obtain the true pillar height from this method. The relatively small size of the probe diameter compared to that of the pillars allows us to obtain a reliable result by engaging only one single pillar. This is confirmed by the microscope fitted on the AFM before each pull-off measurement, where the tests were carried out at the center of the bio-mimicking surfaces as the initial location.
|
Download:
|
| Fig. 1. (a) Gecko attached to the surface of a smooth object; (b) SEM image of a single seta; (c) SEM image of clusters of spatulae branching out the tip of a single seta; (d) Schematic diagram of the fabrication protocol of the biomimetic composite surfaces. | |

|
Download:
|
| Fig. 2. (a) Schematic diagram of the pull-off test method, inset is the SEM picture of the tipless AFM probe modified with the SiO2 particle; (b) SEM images of the asprepared gecko inspired adhesives (3% Fe3O4 content), upper picture is a top view at 60 degrees tilt while the lower one is a side view; (c) Typical force curves, the blue line represents the approaching curve while the red line represents the retreating curve; (d) AFM images of the surface of pure PDMS pillars with a scan size of 70 × 70 μm2. | |
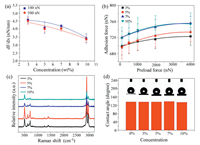
The mechanical properties of the composite biomimetic surface were measured using the modified AFM probe. The adhesion force was tested under nitrogen atmosphere of AFM chamber in order to keep the surface dry and each test was conducted after the sample was stabilized in nitrogen atmosphere for 15 min (temperature: 22±1.2 ℃, relative humidity: 18%±1.0%) [25–27]. The force curves obtained allow us to investigate the Young's Modulus of our samples by employing the DMT model. The slope of the force curve was used to estimate the modulus and the results are shown in Fig. 3a (trend-lines were drawn to serve as a guide). The composite samples showed a decreasing Young's modulus as the concentration of the Fe3O4 nanoparticles increased. The preloading force was further adjusted to obtain the relationship between the adhesion force and the preloading force (Fig. 3b). As the preloading force increased, the adhesion force increased as well. It can be seen that the 3% nanoparticle containing sample showed the lowest adhesion but highest modulus values, while the 10% nanoparticle content composite had the highest adhesion but lowest modulus values. A plausibly explanation is that material with higher modulus has less conformability, such that under same levels of preloading less real contact area is formed, which in turn produces a relatively lower adhesion.
|
Download:
|
| Fig. 3. (a) Slope of force curve as the function of Fe3O4 concentration, under preloading force of 100 nN (blue squares) and 500 nN (red dots), respectively; (b) Adhesion force against preloading force curve between samples of different Fe3O4 concentrations; (c) Raman spectra from four composite surfaces; (d) Contact angle data for biomimetic surfaces with different Fe3O4 concentrations; each data point is the average value of three tests at different locations. Inset pictures above the histogram show the corresponding optical images of the sessile drop. | |
The bio-mimetic surfaces were further investigated to elucidate the cause of the expected enhancement of 'material modulus of the composite samples' by combining hard Fe3O4 nanoparticles with soft PDMS matrix, actually leads to a reduction of the modulus in individual pillars. The Raman spectra of different composite surfaces are shown in Fig. 3c. Here, we implemented identical procedure and chose the focus point right on top of the pillars (excitation laser wavelength of 532 nm, at a room temperature of 25 ℃). The peaks near 490.4 cm-1 correspond to the Si-O-Si bands and the other two peaks around 700 cm-1 are Si—CH3 symmetric bands as well as Si-C symmetric bands, respectively. The CH3 symmetric bend and asymmetric bend bands are attributable to the peaks near 1264.5 cm-1 and 1411.7 cm-1, respectively. The highest peaks that appear at 2906.6 cm-1 and 2965.1 cm-1 are the CH3 symmetric stretch band and CH3 asymmetric stretch band, respectively. The peaks are consistent with the Raman spectra of polydimethylsiloxane [28]. No significant chemical changes were observed between the Fe3O4 and PDMS material, although some secondary interaction changes might present. The magnetic nanoparticles dispersed in the polymer matrix were homogeneous throughout the entire mixture and did not show any obvious peaks in the Raman spectra. The increase of the concentration of the magnetic nanoparticles caused a decrease in the intensity of the peaks, indicating the increase of the Fe3O4 content in the individual pillars and thus the adhesion and self-cleaning properties of the biomimic surfaces were increased, which will be explained in details in the following simulation section.
The gecko toe pad has extremely high hydrophobicity and many synthetic adhesives were fabricated using hydrophobic materials. The surface wettability of the as-prepared composites was also worth exploring. The sessile drop contact angle measurements were conducted, and samples with different concentrations all showed a similar contact angle value of nearly 135°, as shown in Fig. 3d. Upper images in Fig. 3d show the optical images of the water droplet on the composite surfaces. The air pockets between the fiber arrays prevented the liquid from penetrating, and thus created a Cassie state and sustained almost super hydrophobicity. It is demonstrated that the nanoparticles dispersed in the PDMS adhesive influenced the topography of the adhesive surface (Fig. S1 in Supporting information). Notably by understanding the role of Fe3O4 content on modulus of the individual pillars, if the concentration could be controlled in the longitudinal direction, we can make functionally graded pillars that have varying levels of conformability, which help to effectively collect wet or dry adhesion forces on rough surfaces without building sophisticated levels of structural hierarchy.
To analyze how the Fe3O4 nanoparticles were distributed in the micropillar surfaces, we subjected the samples to SEM. Figs. S2a-d (Supporting information) show the results of four different samples: concentrations of 0%, 3%, 5%, 7% and 10% Fe3O4 nanospheres, respectively. The green, blue and the red dot signals to the right correspond to the elements of (ⅰ) silicon, (ⅱ) oxygen and (ⅲ) iron, respectively. Silicon and oxygen are the basic elements of PDMS, and the mapping images displayed a similar morphology of the pillars, indicating that the two elements in the material were evenly distributed. The mapping images of the Fe element (component for the magnetic nanoparticles) revealed an enhancement of the elemental signal as the concentration level increased. No substantial aggregation and/or segregation occurred, indicating a uniform and homogeneous distribution. Moreover, all the composite samples showed a relative homogeneous dispersion not only in the base but also inside the pillar columns. Fig. S3 (Supporting information) also shows an EDS image of the pillars containing 10% magnetic nanoparticles, which supports this conclusion.
Silicon dioxide microspheres approximately 6 μm in diameter were used to simulate the contaminants. We employed house-made testing apparatus to calculate the number of dislodged particles to estimate the self-cleaning rate/capability of four fabricated composite adhesives. An LDP procedure, that is loading, dragging then pulling (Fig. S4 in Supporting information), was implemented to simulate real gecko toe peeling motion in an open environment (temperature: 25±1 ℃). As shown in Fig. 4, the number of dislodged microspheres increased as the LDP movement was imposed repetitively (each data point is the mean value of three trials). The first five steps played a major role, which resulted cleaning almost 90% of the initially attached particles. The contaminants seem to not fall after six or more steps. The 5% and 10% nanoparticle samples showed a relative high drop of microspheres in the beginning and had a dislodging rate of up to 24.2% and 24.6% with only four steps, respectively. The surface morphology did not change after the self-cleaning test. A soft pillar provided an improved adhesion to contaminants while also being more difficult to clean. When a self-cleaning LDP movement was imposed, the microspheres had more opportunities to roll into the spaces between the pillars (Fig. 4b, inset ⅲ), instead of dropping off the adhesive surfaces. As the concentration ratio increases, the modulus of the composite surface pillar decreases. A previous study indicated that the Young' modulus exhibits an increasing trend with the increase of hard embedded particle concentrations in a Shore A method, which has a larger contact area accompanied with a much higher pressure. This resulted in much higher pressure attributed to the hard components.

|
Download:
|
| Fig. 4. (a) Schematic diagram of LDP self-cleaning tests mimicking the gecko toe peeling motion; (b) Histogram of the percentage of the particles dislodged as the step increased in the self-cleaning tests. Inset SEM pictures (ⅰ), (ⅱ) and (ⅲ) represent the initial fully contaminated surface, the state after four steps and nearly a stabilized state after seven steps, respectively (Scale bar - 25 μm); (c) The schematics of the mechanism of changes in Young's module and adhesion force; (d) Dimethyl siloxane polymerase chain, three dimethyl siloxane polymerase chain jointed with one Fe, two dinethylsiloxane polymerase chain jointed with one Fe. Blue, red, gold, brown and pink balls denote Si, O, Fe, C and H atoms, respectively. | |
The pillar modulus showed a reversed trend when compared to the previous study. The underlying mechanism of this has been systemically studied. The Raman spectra and contact angle test show that there were no chemical changes that occurred in the composites, and the chemical properties of the pattern and top surface were almost identical. By way of changing the preloading force to obtain the adhesion data, we found that the adhesion force increased as the preloading force increased. The relationship between the four composites was consistent with their modulus difference. One hypothesis is that the Fe element and PDMS form a coordinate complex, thus influence the Young's modulus and in turn the adhesion properties of the composite. The proposed mechanism is schematically shown in Fig. 4c. Since Si and Fe ion are hard to form stable bonding, the gap between PDMS and Fe ion could be responsible for the decreased manifested in the composite's Young's modulus. This means it is possible to produce a robust self-cleaning surface of a relatively high adhesion on various surfaces. It is speculated that the Fe3O4 nanoparticles within the polymer rein mix possibly cause a reduction in polymerization and act as blockages to obstruct the load transfer in the polymer chains and thus lead to the enhanced of self-cleaning property of the composite.
To validate our hypothesis, we employed DFT simulations to study the connection mechanism of dimethyl siloxane polymerase chain generated by Fe. The optimized structures are shown in Fig. 4d. We use four dimethyl siloxane polymerase chain stands for the PDMS matrix molecule structure. The Fe ion could serve as a joint point connecting dimethyl siloxane polymerase chain as cross-linked structure. We can find the ion bond with three oxygen atoms with bond length of 1.84 Å or with two oxygen atoms with bond length of 1.69 Å. The formation energies of joint structure are -7.37 eV and -11.21 eV for two joint-chain and three joint-chain dimethylsiloxane polymersase, respectively, which is lower than the formation energy of non-joint dimethylsiloxane polymersase chain (-3.24 eV). Therefore, we could infer that the joint structure could form favorably based on thermodynamics. Compared with the dimethylsiloxane polymersase chain, the joint polymersase chain show higher degree of cross-linking, and less freedom of movement. During a LDP process, the degree of crosslinking in Fe3O4/PDMS composites would be varying due to the fact that a different formation energy is required for the non-joint, two-joint-chain and three-joint-chain dimethylsiloxane structures. In the loading and dragging step, the internal energy of the Fe3O4/PDMS increases, favorable to the forming of non-joint-chain dinethylsi-loxane structures, therefore, the modulus of the structure may decrease and adhesion increases [29]. However, in the pulling process, the internal energy of the Fe3O4/PDMS structure decreases, as a result, the two-joint-chain and three-joint-chain dimethylsiloxane could be favored, leading to a recover of the modulus and improved self-cleaning performance [30]. It also explains that the biomimetic materials we use do not change shape after the self-cleaning test.
Thus, loading more Fe3O4 in the PDMS matrix will result in a decrease in pillar modulus, in other words increase in conform-ability to the flat target surfaces. This favors higher adhesion. Meanwhile, the load transferring capability decreases as concen-tration of Fe3O4 is increased from 0%–10%. When there are dirt particles present in between the adhesive pillar and flat surface, the adhesion of dirt particles to the pillar (clogging phenomenon) will be alleviated during each LDP cycle due to 1) the smaller size of the dirt relative to that of pillar and 2) the mismatch of the localized modulus close to and far away from the Fe3O4 region. For samples with higher Fe3O4 concentration, during each LDP, the clogging/attached dirt particles would have a higher chance to interact with the hard Fe3O4 region at the tiptop, which explains the better self-cleaning capabilities measured in our experiment. This hypothesis was further investigated using AFM. The results of the root-mean-square roughness (RMS) indicated that the increased concentration of nanoparticles caused a slight increase of roughness of the top surfaces of the pillars (Fig. S1). In the preloading process, as the internal energy of Fe3O4/PDMS increases, formation of non-joint-chain dinethylsiloxane struc-tures becomes favorable. As a result, the modulus of the structure is expected to decrease and thus lead to an increase in surface contact areas, in which stronger adhesion forces can be found. Meanwhile, the self-cleaning capabilities were improved.
In previous study, Lee and Fearing fabricated a dry self-cleaning adhesive using a high-aspect-ratio fibrillar polypropylene (PP) array with self-cleaning rate up to 33% with 30 steps [31, 32]. In contrast to their work, we demonstrated a composite/hybrid surface that has a self-cleaning capability up to 24.5% within only 4 steps and with enhanced adhesion property. The fabrication of the polymer-inorganic particles composite fibrillar arrays with both enhanced adhesion and self-cleaning properties open a new route to make high performance multifunctional dry contact self-cleaning surfaces that have many practical applications in energy, biomedical and engineering fields.
This study demonstrates a facile way to modulate the modulus of the gecko-inspired self-cleaning surfaces by distributing inorganic magnetic nanoparticles into the polymeric micropillars. The composites were judiciously investigated and characterized with AFM, Raman spectroscopy, contact angle measurements, and SEM incorporated with element mapping. We proposed that the modulus change is caused by a change of the polymerization due to the presence of the Fe3O4 nanoparticles, which are evident in the EDS and RMS experimental results. The relatively softer pillars with high concentration of Fe3O4 distributed, in our study, were demonstrated to have a both increased adhesion and self-cleaning capability. This work provides a new method to precisely regulate the stiffness of the micropillar and improve the adhesion and self-cleaning capabilities of biomimetic surfaces all at once. This work has important implications in fabricating robust smart and multifunctional adhesives, as well as gecko-inspired surfaces with high performance dry contact self-cleaning capabilities.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 51575528, 51875577, 51704243), Beijing Nova Program (No. Z171100001117058), Tribology Science Fund of State Key Laboratory of Tribology (No. SKLTKF16A06), Science Foundation of China University of Petroleum (No. 2462019QNXZ02).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.07.007.
| [1] |
J. Liu, L. Wang, S. Xi, et al., Langmuir 33 (2017) 519-526. DOI:10.1021/acs.langmuir.6b03282 |
| [2] |
S. Tian, X. Dong, T. Wang, et al., Langmuir 34 (2018) 13882-13887. DOI:10.1021/acs.langmuir.8b03151 |
| [3] |
T. Wang, S. Tian, G. Li, et al., J. Nat. Gas Sci. Eng. 50 (2018) 181-188. DOI:10.1016/j.jngse.2017.12.002 |
| [4] |
T. Wang, S. Tian, G. Li, et al., J. Phys. Chem. C 122 (2018) 17009-17018. DOI:10.1021/acs.jpcc.8b02061 |
| [5] |
D.M. Jarvie, R.J. Hill, T.E. Ruble, et al., AAPG Bull. 91 (2007) 475-499. DOI:10.1306/12190606068 |
| [6] |
D.J.K. Ross, Marc Bustin R., Mar. Petrol. Geol. 26 (2009) 916-927. DOI:10.1016/j.marpetgeo.2008.06.004 |
| [7] |
S.R. Kelemen, M. Afeworki, M.L. Gorbaty, et al., Energy Fuel 21 (2007) 1548-1561. DOI:10.1021/ef060321h |
| [8] |
M. Djuricic, R.C. Murphy, D. Vitorovic, et al., Geochim. Cosmochim. Acta 35 (1971) 1201-1207. DOI:10.1016/0016-7037(71)90111-6 |
| [9] |
F. Javadpour, M.M. Farshi, M. Amrein, J. Can. Pet. Technol. 51 (2012) 236-243. DOI:10.2118/161015-PA |
| [10] |
Q. Wang, Y. Hou, W. Wu, et al., Fuel Process. Technol. 166 (2017) 30-40. DOI:10.1016/j.fuproc.2017.05.024 |
| [11] |
S.R. Kelemen, M. Afeworki, M.L. Gorbaty, et al., Energy Fuel 16 (2002) 1507-1515. DOI:10.1021/ef0200828 |
| [12] |
J. Tong, X. Han, S. Wang, et al., Energy Fuel 25 (2011) 4006-4013. DOI:10.1021/ef200738p |
| [13] |
A. Dazzi, F. Glotin, R. Carminati, J. Appl. Phys. 107 (2010) 124519. DOI:10.1063/1.3429214 |
| [14] |
A. Centrone, Ann. Rev. Anal. Chem. 8 (2015) 101-126. DOI:10.1146/annurev-anchem-071114-040435 |
| [15] |
J. Yang, J. Hatcherian, P.C. Hackley, et al., Nat. Commun. 8 (2017) 2179. DOI:10.1038/s41467-017-02254-0 |
| [16] |
A. Dazzi, C.B. Prater, Q. Hu, et al., Appl. Spectrosc. 66 (2012) 1365-1384. DOI:10.1366/12-06804 |
| [17] |
A. Dazzi, C.B. Prater, Chem. Rev. 117 (2017) 5146-5173. DOI:10.1021/acs.chemrev.6b00448 |
| [18] |
P.C. Painter, R.W. Snyder, M. Starsinic, et al., Appl. Spectrosc. 35 (1981) 475-485. DOI:10.1366/0003702814732256 |
| [19] |
E. Riedo, F. Lévy, H. Brune, Phys. Rev. Lett. 88 (2002) 185505. DOI:10.1103/PhysRevLett.88.185505 |
| [20] |
Y. Wang, X. Gong, J. Mater. Chem. A 5 (2017) 3759-3773. DOI:10.1039/C6TA10474F |
| [21] |
Y. Wang, X. Gong, Adv. Mater. Interfaces 4 (2017) 1700190. DOI:10.1002/admi.201700190 |
| [22] |
X. Gong, Y. Wang, T. Kuang, ACS Sustain. Chem. Eng. 5 (2017) 11204-11214. DOI:10.1021/acssuschemeng.7b03613 |
| [23] |
S.Y. Krylov, K.B. Jinesh, H. Valk, et al., Phys. Rev. E 71 (2005) 065101. |
| [24] |
X. Zhao, M. Hamilton, W.G. Sawyer, et al., Tribol. Lett. 27 (2007) 113-117. DOI:10.1007/s11249-007-9220-2 |
| [25] |
L. Liu, J. Lu, Y. Zhang, et al., Green Chem. 21 (2019) 526-537. DOI:10.1039/C8GC03560A |
| [26] |
H. Liang, S. Wang, H. He, et al., Ind. Crops Prod. 122 (2018) 182-189. DOI:10.1016/j.indcrop.2018.05.079 |
| [27] |
L. Liu, H. Liang, J. Zhang, et al., J. Clean. Prod. 195 (2018) 786-795. DOI:10.1016/j.jclepro.2018.05.216 |
| [28] |
A. Schirmeisen, L. Jansen, H. Hölscher, et al., Appl. Phys. Lett. 88 (2006) 123108. DOI:10.1063/1.2187575 |
| [29] |
I.Y. Jeon, J.B. Baek, Materials 3 (2010) 3654-3674. DOI:10.3390/ma3063654 |
| [30] |
Y. Tian, Z. Zhao, G. Zaghi, ACS Appl. Mater. Interface 7 (2015) 13232-13237. DOI:10.1021/acsami.5b03301 |
| [31] |
J. Lee, R.S. Fearing, Langmuir 24 (2008) 10587-10591. DOI:10.1021/la8021485 |
| [32] |
C. Zhang, D.A. Mcadams, J.C. Grunlan, Adv. Mater. 28 (2016) 6292-6321. DOI:10.1002/adma.201505555 |
 2019, Vol. 30
2019, Vol. 30 

