Rare earth elements appeared at the end of the 18th century, and on the basis of their unique electronic structure and physical and chemical properties, they can be divided into heavy, middle and light rare earth elements. The special position in the periodic table where the rare earth elements are found determines their many excellent properties. Specifically, they have a special 4f electronic structure layer that results in a larger atomic magnetic moment and a spin coupling ability, which have led to their wide use in agriculture, metallurgy, petrochemical industry, and optoelectronics, known as the "gold industry" and an "industrial vitamin" [1–4].
China has an abundance of rare earth resources, and is the leading country for rare earths storage power for the world's reserves, which consist of 154 million tons [5, 6]. In recent years, with the development of the pool leaching, heap leaching and in-situ leaching mining technologies [7], using rare earth resources has caused several waste of resources. The data shows that the pool leaching process of rare earth resources has a recovery rate of about 30% producing 1 t of rare earth oxide via pool leaching produces about 1000 t of wastewater that contains 50 mg/L of rare earth ions because of relatively backward technology [8–10]. Thus, the preparation of an environmentally friendly, efficient and recoverable adsorbent is of great significance to recycling rare earth resources.
In the 1992, a new kind of molecular sieve in the M41S family, having a diameter in the range of 1.5–10 nm, was successfully synthesized by the Mobil ℃ompany [11, 12]. The MCM-41 mesoporous molecular sieve possesses a six-part ordered mesoporous structure that is uniform and controllable, the specific surface area and the inner surface is easily modified with organic groups. Thus, it is an ideal kind of matrix [13–15], and it has a wide range of applications in the fields of catalysis, adsorption, and separation.
In this paper, the sulfhydryl MCM-41 (SH-MCM-41) molecular sieve was prepared via a hydrothermal synthesis method. First, the pure MCM-41 mesoporous material was synthesized, and then using an alkaline solvent, the MCM-41 mesoporous material was alkylated by 3-mercaptopropyltrimethoxysilane to bring the mercapto groups into the channel of MCM-41. Application of SH-MCM-41 for adsorption of La3+, Gd3+ and Yb3+ from rare earth wastewater was examined, and the stripping experiment was explored.
MCM-41 mesoporous silica was prepared in a classical alkaline medium using conventional hydrothermal crystallization [16]. 1 g hexadecyl trimethyl ammonium bromide (CTAB) was added to a solution of ammonium hydroxide. The mixture above was stirred for 1 h at 60 ℃. 0.025 mol tetraethoxysilane (TEOS) was added dropwise to the gel under vigorous stirring. A certain ratio of 3-mercaptopropyltrimethoxysilane (MPTES) was added to the white solution after 1 h. After stirring for 6 h at 60 ℃, the resulting homogeneous mixture was crystallized under static hydrothermal conditions at room temperature for 72 h. The material was filtered and washed with distilled water, and dried at 100 ℃. It was extracted in absolute ethyl alcohol at 80 ℃ for 6 h to remove the organic template; the material was cooled to room temperature, filtered, washed and dried at 110 ℃ overnight.
X-ray powder diffraction patterns were operated at an acceleration voltage of 40 kV using Cu Kα radiation, and the data were recorded in the 2θ range of 1°~10°. The Fourier transform infrared spectra for the unmodified and modified samples were measured on a 550Ⅱ instrument. The carbon, hydrogen and nitrogen contents were analyzed using an automatic analyzer (EA, ELIII). Adsorption-desorption isotherms of the synthesized samples were measured using a micromeritics model ASAP-2020 sorptometer to determine the average pore diameter and total pore volume. The pore size distributions were calculated using the Barrett-Joyner-Halenda. A TGA4000 thermogravimetric analyzer (PE companies in the United States)was used to conduct the thermal analysis, under an atmosphere of air, in the temperature range of 20–800 ℃, with a heating rate of 10 ℃/min. The structure and morphology of samples were examined using scanning electron microscopy (SEM, FEI Quanta200 F) and transmission electron microscopy (TEM, JEM-2100). The concentrations of rare earth ions in the solutions were measured using ultraviolet-visible spectrophotometry (UV-2550).
The adsorption experiments were performed using a batch technique to get the adsorption equilibrium and rate dates. For single solute adsorption studies, 5–40 mg of the adsorbent was added to a series of 30 mL conical flasks that had gradient La3+, Gd3+ and Yb3+ concentrations. The conical flasks were then kept on a rotary shaker at different ambient temperatures for 1 h to attain adsorption equilibrium. At the end of the adsorption process, the adsorbent was filtered, and the concentrations of the La3+, Gd3+, and Yb3+ in the residual solution were analyzed using ultraviolet-visible spectrophotometry at a wavelength of 655 nm. The equilibrium adsorption capacity of the adsorbent was calculated according to the following equations (Eqs. 1 and 2) [17]:
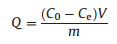
|
(1) |
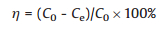
|
(2) |
where Qe (mg/g) is the equilibrium adsorption capacity; C0 and Ce(mg/L) are the initial and equilibrium concentrations, respectively, of the La3+, Gd3+and Yb3+; V (L) is the volume of the solution; m (g) is the mass of the adsorbent, and η is the adsorption rate of the rare earth ions.
To estimate the recoveries of the La3+, Gd3+ and Yb3+ from SH-MCM-41, desorption experiments with hydrochloric acid solution were performed. First, the absorbent, which was already saturated, was put into an adsorption column, and the adsorption column was washed with a small amount of deionized water to remove impurity ions. Then 2 mol/L solution of hydrochloric acid was used to wash the adsorption column with a flow velocity of 1 mL/min in the whole process. The elution tail liquid was accessed every 2 min to calculate the La3+, Gd3+and Yb3+ concentrations in the solution and to calculate the adsorption rates. The processes of adsorption and desorption were repeated to observe the reusability of the absorbent
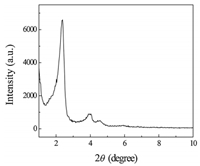
Fig. 1 shows the X-ray diffraction (XRD) patterns of SH-MCM-41 and shows three diffraction peaks that can be indexed to (100), (110) and (200) in the 2θ range from 1° to 10°. A strong diffraction peak in the low-angle region (2θ= 2.18°) is associated with the (100) reflection of the hexagonal cell. Except for this intense peak, we also observe two additional weak peaks (2θ= 3.88° and 4.56°) that can be indexed to the (110) and (200) reflections of the typical hexagonal cell, this is consistent with the typical characteristics of the MCM-41 material reported in the literature [18]. After modification, the (100) diffraction peak shifts to the direction of the larger angle, indicating that the mercapto-grafted sample has a high degree of crystallinity and that the modification process does not affect the structure of the ordered mesoporous MCM-41. By calculation 
|
Download:
|
| Fig. 1. XRD pattern of SH-MCM-41. | |
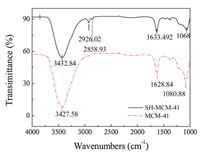
The FT-IR spectra of the MCM-41and SH-MCM-41 samples are shown in Fig. 2. There are significant differences in the mesoporous materials before and after functionalization. The broad absorption band in the region of about 3430 cm-1 can be attributed to the stretching of the 
|
Download:
|
| Fig. 2. FTIR spectra of MCM-41 and SH-MCM-41. | |
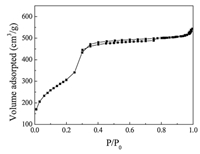
Fig. 3 shows the nitrogen adsorption-desorption isotherms of SH-MCM-41. According to IUPAC regulations [21], mesoporous materials are generally characterized by isothermal adsorption-desorption (Ⅳ) type and usually have an adsorption hysteresis loop. The variation curve is divided into three stages. First, in the low pressure stage, N2 in the inner mesoporous surface did not have a saturated adsorption monolayer, and the adsorption amount increased gently. Second, with an increase in the relative system pressure, N2 underwent the capillary condensation phenomenon in the tunnel. Third, when the relative pressure was 0.3, the adsorption quantity spurted, the isothermal adsorption curve rose sharply, and there was an obvious turning point. After that point, the platform shows that N2 adsorption reached equilibrium in the mesoporous material. The SH-MCM-41 sample we prepared was a porous material with surface area of 956.0 m2/g and average pore size of 2.1 nm. The synthesized mesoporous structure had slightly larger micropores, which are helpful for making contact between active adsorption sites and the small diameter rare earth ions, as well as for promoting the adsorption process.
|
Download:
|
| Fig. 3. Adsorption-desorption isotherms of SH-MCM-41. | |
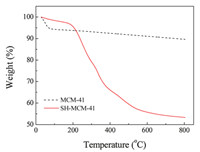
Fig. 4 shows the TG analysis of MCM-41 and SH-MCM-41. When the test temperature was increased from room temperature to 200 ℃, the free adsorption water molecules in the pore and crystallization water were gradually removed. According to the TG curve, the MCM-41 wt loss was about 6.6%, and the SH-MCM-41 wt loss was about 3%. When the temperature was further increased to 600 ℃, there was residual surfactant in the mesoporous materials from decomposition by burning, and the surface silicon hydroxyl condensation formed a silicon-oxygen bond. For SH-MCM-41, the mercapto groups grafted in the channel of the mesoporous material were also resolved in this range of temperatures [22]. According to the TG curve, the MCM-41 wt loss was about 2%, and the SH-MCM-41 wt loss was about 4%.
|
Download:
|
| Fig. 4. TG analysis of MCM-41 and SH-MCM-41. | |
Fig. 5A shows the scanning electron microscopy images of SH-MCM-41. The images clearly show that the mesoporous samples preserved the regular hexagon morphology [23]. According to the high resolution TEM images shown in Fig. 5B, the size distributions of the hexagon particles of the samples were uniform.

|
Download:
|
| Fig. 5. SEM (A) and TEM (B) images of SH-MCM-41. | |
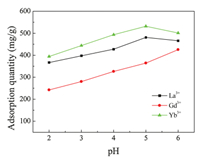
The pH of an aqueous medium is one of the most important factors in the adsorption process [24]. To detect the effects of pH on the adsorption capacity of SH-MCM-41, rare earth solutions were prepared at different pH values ranging from 2.0 to 6.0, NH3·H2O and HCl solutions were used to adjust the pH of the solutions. The experimental results of the adsorption of the La3+, Gd3+ and Yb3+ on SH-MCM-41 are shown in Fig. 6. It was found that the adsorption capacity increased when the pH value increased from 2 to 5. This suggests that the adsorption of the La3+, Gd3+ and Yb3+ depends on the pH of the solution, which influences the static electricity of the La3+, Gd3+ and Yb3+ bound to mercapto groups. When the pH value is small, the number of binding sites that are available for the adsorption of the La3+, Gd3+ and Yb3+ decreased. In other words, the protons in the solution compete with the La3+, Gd3+ and Yb3+ adsorbing onto the SH-MCM-41material. ℃onsequently, the adsorption capacity of the La3+, Gd3+ and Yb3+ decreased at low pH. As the pH of the solution increased, the adsorption quantities also increased. When the pH value was higher, the rare earth ions precipitated because of high concentrations of OH- ions in the aqueous solution, causing a decrease in the adsorption capacity. In this study, the best solution pH value for La3+ and Yb3+ is 5.0, and that for Gd3+ is 6.0.
|
Download:
|
| Fig. 6. Effect of pH on adsorption. | |
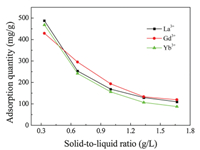
Under the same experimental conditions, the dosage of the adsorbent affects the saturated adsorption quantity of rare earth ions. Fig. 7 shows the effects of the solid-liquid ratio on the adsorption reaction (pH value for La3+ and Yb3+ is 5.0, and that for Gd3+ is 6.0. Initial concentration is 400 mg/L). With an increase in the solid-liquid ratio, the dispersion of the adsorbent in solution is worse, causing the active sites to cohere to one another, and the utilization rate is reduced, which decreases the adsorption quantity of the rare earth ions. At the same temperature, the adsorption effects of the La3+ and Yb3+ were slightly better than the Gd3+. This is mainly because of the different coordination abilities between S and the rare earth ions. The most common coordination number of the La3+ and Yb3+ is 8, and thus they can be more effectively used by S in coordination interactions. In contrast, the coordination number of the Gd3+ is 9, and the utilization of S is worse. In addition, the Gd3+ need more S to form stable rare earth complexes, so S tends to coordinate more with the La3+ and Yb3+, increasing the adsorption quantities of this ions when the mounts of S is constant.
|
Download:
|
| Fig. 7. Effect of solid-liquid ratio on adsorption. | |
Mesoporous materials can be used as good adsorption carriers because of their neat and orderly structure, and their stable skeleton. The inner surface is easily functionalized, and so a variety of adsorbents can be synthesized with different surface properties. Studies have shown that [25, 26]: the grafting amount of S is closely related to the adsorption quantity of rare earth ions. This paper prepared mesoporous materials with different amounts of -SH, and the modification rates were determined via elemental analysis (EA). The effects of the grafted amount on the adsorption of the La3+, Gd3+ and Yb3+ was studied, and the results are shown in Table S1 (Supporting information).
The data indicates that the adsorption capacities of the La3+, Gd3+ and Yb3+ increased with an increasing amount of grafted mercapto groups. This increase of adsorption capacity is because of the coordinate interaction of S with the La3+, Gd3+ and Yb3+. On the other hand, when the added MPTES was greater than 4.78 10-4 mol/g, the mesoporous channel became jammed, causing a decrease in the specific surface area. Thus, there is a negative effect on the adsorption capacity. In this study, the best amount of MPTES is 4.78 10-4 mol, and the maximum adsorption capacities of the La3+, Gd3+ and Yb3+ are 560.56 mg/g, 467.60 mg/g and 540.68 mg/g, respectively.
Equilibrium studies were carried out to detect the equilibrium constant and the adsorption capacity of the SH-MCM-41 sample. The distribution of the absorbent at the interface of the solid and liquid phases is a rule of the distribution coefficient in the process of adsorption, and it can be expressed by the Langmuir and Freundlich equations. The adsorption isotherms were obtained under different temperatures at the optimum adsorption conditions. In this study, the obtained experimental equilibrium adsorption data are fitted using the Langmuir (Eq. 3) [27] and Freundlich (Eq. 4) [28] isotherms.
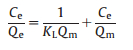
|
(3) |
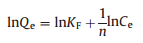
|
(4) |
where Qe (mg/g) is the adsorption capacity at equilibrium; Ce (mg/L) is the equilibrium concentration of the rare earth ions, and Qm (mg/g) is the maximum monolayer adsorption capacity. KF is a Freundlich isotherm constant and the slope 1/n, ranges from 0 to 1, indicating the degree of nonlinearity between the solution concentration and the adsorption. KL is a constant related to the free energy of adsorption. The isotherm data were linearized using the Freundlich and Langmuir equations and are provided in Tables S2-S4 (Supoprting information) for La3+, Gd3+ and Yb3+, respectively.
It is clear that the adsorption isotherms are better fit using the Freundlich isotherm models than with the Langmuir isotherm models (evidenced from R2 > 0.91). This may be ascribed to the disorder in the pore structure of SH-MCM-41, which is induced by the La3+, Gd3+ and Yb3+ adsorption, and as a consequence of this, the adsorption solution may become heterogeneous and the adsorption isotherms are type Ⅳ instead of type I. In this case, the Freundlich isotherm model may be more appropriate than Langmuir isotherm model for describing the adsorption process. According to the theory of Freundlich isotherms, the KF value indicates the adsorption capability and the n value reflects the adsorption intensity. In this experiment, all n > 1 indicate the preferential adsorption.
The adsorption process is always accompanied by heat evolution since the adsorbents are more stable on the adsorbent surface than in solution. To explore the adsorption thermodynamics using Freundlich isotherm model, the parameters were calculated by the following equations [29]:

|
(5) |
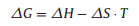
|
(6) |
where KF is a Freundlich isotherm constant for the system. R is the gas constant, and T is the temperature of the solution (in Kelvin). The results of the calculations are shown in Table S5 (Supporting information).
As seen in Table S5, the △H values of La3+ and Gd3+ are negative in the adsorption reaction, indicating that the adsorption process is exothermic, and heating thus opposes the adsorption. In contrast, the △H value of Yb3+ is positive, meaning that the adsorption process is endothermic. The △S values of La3+ and Gd3+ are positive, indicating that the molecules and ions become more chaotic on the solid-liquid interface, and that the entropy of the system increases with the adsorption process. Thus the data shows that the process is "entropy driven"; the △G values are all negative, indicating that the adsorption reactions of La3+, Gd3+ and Yb3+ on SH-MCM-41 can be spontaneous.
The effects of the contact time of the La3+, Gd3+ and Yb3+ adsorbed on SH-MCM-41were measured at the optimum initial concentration (250 mg/L) and temperature (25 ℃). The controlling mechanisms of the adsorption process were investigated according to pseudo-first-order and pseudo-second-order kinetics equations. The pseudo-first-order kinetics model is given as (Eq. 7), and the pseudo-second-order kinetics model is given as (Eq. 8) [30, 31]:
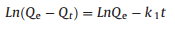
|
(7) |
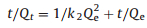
|
(8) |
where Qe (mg/g) and Qt (mg/g) are the adsorption capacities of the La3+, Gd3+ and Yb3+ at equilibrium and time t, respectively. k1 (min-1) is the rate constant of the first-order adsorption, and k2 (g mg-1 min-1) is the rate constant of the second-order adsorption. The parameters of the pseudo-first-order and pseudo-second-order adsorption kinetics models are listed in Table S6 (Supporting information).
It is evident that the coefficient values of the pseudo-second-order model exceeded 0.999, and that of the pseudo-first-order model exceeded 0.810. The calculated adsorption capacities obtained from the pseudo-second-order model were as good as the experimental adsorption capacity. Thus, the pseudo-second-order model is more suitable for describing the kinetics of the La3+, Gd3+ and Yb3+, implying that the adsorption process on SH-MCM-41 may take place via a chemical process involving valence forces via an exchange or sharing of electrons.
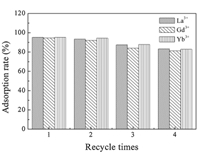
Since the recovery of the absorbents is of great importance in adsorption systems, experiments regarding the desorption of the La3+, Gd3+ and Yb3+ and the recycling of the SH-MCM-41 absorbent were conducted. The results are shown in Fig. 8.
|
Download:
|
| Fig. 8. Results of desorption and reusability on SH-MCM-41. | |
The La3+, Gd3+ and Yb3+ adsorbed on the SH-MCM-41 absorbent were effectively eluted using 2 mol/L HCl as an eluent. In the case of this experiment, the desorption efficiency values were as high as 93.4%, 92.1% and 95.3% for La3+, Gd3+ and Yb3+, respectively. The adsorption-desorption cycle results indicate that the absorbent could be reused up to 4 times without a significant change in the elution rate. Therefore, SH-MCM-41 is promising in practical applications for adsorbing La3+, Gd3+ and Yb3+ from rare earth wastewater.
Adsorption of rare earth ions from rare earth wastewater via the adsorption method is a fundamental study. A kind of functionalized material was synthesized, and named SH-MCM-41. SH-MCM-41 was examined in an adsorption experiment to investigate the adsorption capacities of La3+, Gd3+ and Yb3+. The results suggest that the solution pH value affects the adsorption process significantly, and in this study, the optimized solution pH value for La3+ and Yb3+ is 5.0, and that for Gd3+ is 6.0. The Freundlich isotherm model is more suitable than the Langmuir isotherm model. The maximum adsorption capacities of the La3+, Gd3+ and Yb3+ are 560.56 mg/g, 467.60 mg/g and 540.68 mg/g, respectively. The adsorption kinetic data for the SH-MCM-41 adsorbent matched the pseudo-second-order kinetics model well. The adsorption process is a spontaneous exothermic reaction because it is "entropy driven". Desorption and recycling experiments show that the SH-MCM-41 adsorbent had a strong regeneration ability, and thus it has potential application in enriching and recycling rare earth ions from low concentration rare earth wastewater. In summary, the organically modified silica-based mesoporous material opens a new efficient method for ion adsorption in future applications.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.05.056.
| [1] |
Y.W. Huang, B. Xiao, J. Conserv. Util. Mineral Resour. 6 (2018) 51-54. |
| [2] |
Y. Xu, Y.Q. Zhang, X.W. Huang, J. Chin. Soc. Rare Earths 30 (2012) 538-544. |
| [3] |
S. Gasser, M.I. Aly, Int. J. Mineral Process. 121 (2013) 31-38. DOI:10.1016/j.minpro.2013.02.012 |
| [4] |
H. Yang, J. Chin. Rare Earths 31 (2012) 87-92. |
| [5] |
H. Zhou, P. Zhang, J. Resour. Ind. 13 (2011) 95-100. |
| [6] |
Y.X. Li, L. Zhang, X.M. Zhou, J. Chinese Rare Earths 31 (2010) 61-69. |
| [7] |
W.M. Huang, X.W. Huang, RE Separation and Attainment of High Purity Products in China, The Second Rare Earths Conference in Rio De Janeiro (1998).
|
| [8] |
C.G. Luo, X.P. Luo, J. Su, et al., J. Metal Mine. 6 (2014) 91-97. |
| [9] |
J. Sun, J.W. Li, H.X. Xu, J. Environ. Impact Assess. 38 (2016) 20-23. |
| [10] |
Y. Cao, X. Guan, J. China Mining Mag. 25 (2016) 60-63. |
| [11] |
J.S. Beck, J.C. Vartuli, W.J. Roth, et al., J. Am. Chem. Soc. 114 (1992) 10834-10843. DOI:10.1021/ja00053a020 |
| [12] |
C.T. Kresge, M.E. Leonowica, W.J. Roth, Nature 359 (1992) 710-712. DOI:10.1038/359710a0 |
| [13] |
D.P. Quintanilla, I. Hierro, M. Fajardo, Mater. Res. Bull. 42 (2007) 1518-1530. DOI:10.1016/j.materresbull.2006.10.029 |
| [14] |
F. Embert, A. Mehdi, C. Reyé, J. Chem. Mater. 13 (2001) 4542-4549. DOI:10.1021/cm0101982 |
| [15] |
Y. Cao, C.J. Li, Q.Y. Huang, Chin. J. Environ. Eng. 6 (2011) 1315-1320. |
| [16] |
D.Y. Zhao, Q.S. Huo, J.L. Feng, J. Am. Chem. Soc. 120 (1998) 6024-6036. DOI:10.1021/ja974025i |
| [17] |
J. Ponou, L.P. Wang, G. Dodbiba, Chem. Eng. J. 2 (2014) 1070-1081. |
| [18] |
P. Smirnov, T. Yamaguchi, S. Kittaka, J. Phys. Chem. B 104 (2000) 5498-5504. DOI:10.1021/jp994326+ |
| [19] |
A. Benhamou, M. Baudu, Z. Derriche, J. Hazard. Mater. 171 (2009) 1001-1008. DOI:10.1016/j.jhazmat.2009.06.106 |
| [20] |
J.J. Chen, K.M. Fang, Y.Q. Miao, J. Nanosci. Nanotechnol. 11 (2011) 1-8. DOI:10.1166/jnn.2011.3839 |
| [21] |
S. Brunauer, L.S. Deming, W.E. Deming, J. Am. Chem. Soc. 62 (1940) 1723-1732. DOI:10.1021/ja01864a025 |
| [22] |
H. Yang, Synthesis and Adsorption Performance of Functionalized Mesoporous Silicates PhD thesis, Tongji University, Shanghai, 2008.
|
| [23] |
Q.L. Liao, L.M. Zeng, F. Guo, Technol. Water Treat. 9 (2013) 49-52. |
| [24] |
M. Amara, H. Kerdjoudj, Talanta 60 (2003) 991-1001. DOI:10.1016/S0039-9140(03)00155-3 |
| [25] |
A. Walcarius, C. Delacote, Chem. Mater. 15 (2003) 4181-4192. DOI:10.1021/cm031089l |
| [26] |
K.H. Nam, L.L. Tavlarides, Chem. Mater. 17 (2005) 1597-1604. DOI:10.1021/cm048269v |
| [27] |
M. Mazzotti, J. Chromatogr. A 1126 (2006) 311-322. DOI:10.1016/j.chroma.2006.06.022 |
| [28] |
S.J. Allen, G. Mckay, J.F. Porter, J. Colloid Interface Sci. 280 (2004) 322-333. DOI:10.1016/j.jcis.2004.08.078 |
| [29] |
F. Huang, F. Zhang, B. Wang, Chin. J. Appl. Chem. 31 (2014) 1458-1463. |
| [30] |
M. Hartmann, A. Vinu, G. Chandrasekar, Chem. Mater. 17 (2005) 829-833. DOI:10.1021/cm048564f |
| [31] |
B. OepenVon, W. Kordel, Chemosphere 22 (1991) 285-304. DOI:10.1016/0045-6535(91)90318-8 |
 2019, Vol. 30
2019, Vol. 30 

