b State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, China;
c Department of Chemistry, Graduate School of Science, Hiroshima University, Higashi-Hiroshima 739-8526, Japan
Dipyrromethene as a classical bidentate ligand, complexes with one BF2 unit (BODIPY) to show highly strong absorption, high fluorescence quantum yields, and chemical and photochemical stability, thereby finding many applications as fluorescent dyes (Fig. 1a) [1-5]. Directly linked bis-BODIPYs were designed and investigated the linked mode to affect the optical properties [6-8]. Moreover, bis-BODIPY derivative contains a pyrrolopyrrole moiety and provides a good example of the effects of forming a more rigid chromophore DPP-dye [9]. The new pyrrole-BF2-based fluorophore bis(difluoroboron)1, 2-bis((1H-pyrrol-2-yl)methylene)hydrazine (BOPHY) was reported by Ziegler and Jiao et al. [10-13]. This aesthetic symmetric structure is composed of four rings at the same plane, including two BF2 units in six-membered chelate rings in the center and two pyrrole units on the periphery (Fig. 1a). The fluorescence quantum yield for the unmodifed BOPHY is so high to near to be 100%. BOPHY has quickly been attracting increasing interest in applications of energy-transfer cascades, probes, fluorescence imaging reagents, and photodynamic therapy. Recently, Jiao et al. reported the new structural motif for unsymmetrical bis(BF2) fluorophore containing both pyrrole and N-heteroarene derivatives (BOPPY) (Fig. 1a) [14]. Except one defect of short wavelength emission, BOPPYs possess high fluorescence quantum yields, large Stokes shifts, excellent stability and good molar absorptivity. Since structural innovation of BODIPYs and their derivatives have been a key area of concern, the creativity of new structural motif is of great importance. Our recent research interest lies in the novel fluorescent dyes [15, 16, 17-20]. Herein, we report 5, 6, 5, 6-tetracyclic pyrazine/pyrrole-fused unsymmetric bis (BF2) fluorescent dyes (BOPYPYs) for the first time. And, the dimethylamino-containing BOPYPY with the long-wavelength absorption could apply for detecting pH values.
|
Download:
|
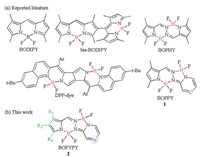
| Fig. 1. Design strategies for 5, 6, 5, 6-tetracyclic unsymmetric bis(BF2) fluorescent dyes (BOPYPYs) by the fusion of a pyrazine moiety. | |
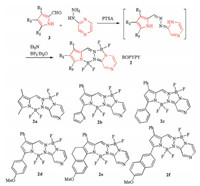
To achieve the long-wavelength absorption dyes, the arylcontaining pyrroles were employed to synthesize BOPYPYs. Pyrrole-2-carboxaldehyde 3 reacted with 1-(pyrazin-2-yl)hydrazine in the presence of p-toluenesulfonic acid (PTSA) to give the precursor, which was followed by the complexation with Et3N-BF3·Et2O to produce the target compounds BOPYPYs 2 (Scheme 1). The structures of BOPYPYs were confirmed by NMR and FTMS (Supporting information). To the best of our knowledge, this is the first synthesis of pyrazine-fused unsymmetric bis(BF2) fluorescent dye BOPYPY.
|
Download:
|
| Scheme 1. Synthesis of fluorescent BOPYPYs 2a-2f. | |
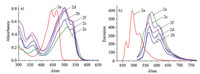
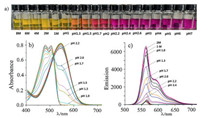
Absorption spectrum of the pyrazine-fused BOPYPY 2a exhibits two main peaks (λabs) at 443 nm and 467 nm, respectively, and its absorption maxima are remarkable bathochromic shifts comparing to those (λabs = 408 and 428 nm) of the pyridine-fused BOPPY 1 reported by Jiao [14]. Due to the different electronegativity between C and N, the discrepant electron withdrawing substitutes between pyridine and pyrazine result in the obvious wavelength difference. BOPYPY 2a emits at 490 nm, 522 nm, and has the obviously large Stokes shifts (79 nm) comparing to the narrow one of the typical BODIPY dye (Table 1). And, the molar extinction coefficients and fluorescence quantum yields between 2a and 1 were comparable. In comparison to 2a, BOPYPYs 2b-2f by the extension of π-conjugation of the corresponding pyrroles, have the long-wavelength absorptions (λabs = 498–546 nm), and their emissions even reach the red region (λem = 560–610 nm). Moreover, BOPYPYs 2b-2f have high molar extinction coefficients, high fluorescence quantum yields, and larger Stokes shifts (96–106 nm) (Fig. 2). Moreover, the computed photophysical data of 2a-2f by TDDFT at the B3LYPG(d) level with Gaussian 09 W (Supporting inforamtion), are in agreement with the experimental data (Table 1).
|
|
Table 1 Photophysical properties of dyes 2a-2f in CH2Cl2 at 293 K. |
|
Download:
|
| Fig. 2. (a) Normalized absorption and (b) fluorescence spectra of BOPYPYs 2a-2f in CH2Cl2 at 293 K. | |
Since the new BOPYPYs were successfully synthesized, their modification and application were investigated. A Knoevenagel reaction of BOPYPY 2a with 4-dimethylaminobenzaldehyde smoothly produced dye 4 bearing the extension of π-conjugation (Scheme 2) [21, 22]. Comparing to these of dyes 2a-2f, dye 4 has a relative long-wavelength absorption (λabs = 546 nm). The most popular strategy for pH-responsive fluorescent sensors takes advantage of intramolecular charge transfer (ICT) or the photoinduced electron transfer (PET) [23, 24]. Dimethylamino group is one of the fragments frequently used for the purpose of ICT. So, herein we explore the response of the dimethylamino-containing BOPYPY 4 to pH values.

|
Download:
|
| Scheme 2. Synthesis of dye 4 by Knoevenagel reaction. | |
Under normal room illumination the photo image of 4 were taken, and remarkable changes from relatively vivid bright colors of 4 with HCl can be easily observed with naked eye (Fig. 3a and Fig. S1 in Supporting information). Upon addition of HCl to BOPYPY 4 bearing the dimethylamino group as a pH-sensitive functionality, 4 could be protonated at the limitative pH value [25, 26]. A stepwise decrease of the absorption intensity was observed in the 546 nm band, and the formation of a new band for 4+H+ at 481 and 503 nm was observed (Fig. 3b). The absorption band of 4+H+ is blue-shifted by about 43 nm compared to that of 4. The fluorescence of 4 is quenched owing to the ICT effect. However, with decreasing pH the emission maxima were shifted to 563 nm (Fig. 3c). A dramatic increase in fluorescence intensity about 20 folds (Φf = 0.41 when treated with HCl to 2 mol/L) (Fig. 3c).
|
Download:
|
| Fig. 3. (a) Photograph of solutions of 2 μmol/L dye 4 at pH 7-1 and 1–8 mol/L of HCl in CH3CN/H2O (1:9, v/v) under normal room illumination. Absorption (b) and florescence (c) spectra (pH 7-1 and 1–8 mol/L of HCl) of 2 μmol/L dye 4 in CH3CN/ H2O (1:9, v/v) as a function of pH. | |
In conclusion, tetracyclic pyrazine/pyrrole-fused unsymmetric bis(BF2) fluorescent dyes (BOPYPYs) were synthesized by reaction of pyrrole-2-carboxaldehyde with 1-(pyrazin-2-yl)hydrazine in the presence of Et3N-BF3·Et2O. The absorption maxima of pyrazine-fused BOPYPY 2a are remarkable bathochromic shifts comparing to those of BOPPY 1, indicating that the discrepant substitute groups between pyridine and pyrazine result in the obvious wavelength difference. The new series of BOPYPYs 2a-2f possess high molar extinction coefficients, high fluorescence quantum yields, and larger Stokes shifts. Moreover, a Knoevenagel reaction of BOPYPY 2a with 4-dimethylaminobenzaldehyde smoothly produced dye 4 bearing the dimethylamino group. BOPYPY 4 as a pH-responsive fuorescent sensor could detect pH value.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21542004), Young and Middle-aged Scientific and Technological Innovation Talents of Shenyang Science and Technology Bureau (No. RC170140), Natural Science Foundation of Liaoning Province (No. 20170540721), Basic research on the application of Industrial Development of Shenyang Science and Technology Bureau (No. 18013027), Liaoning BaiQianWan Talents Program, and the Distinguished Professor Project of Liaoning province (No. 20183532). We thank the Chinese Scholarship Council (No. 20183058) for financial support. We also thank Prof. Yohsuke Yamamoto and Dr Rong Shang (Hiroshima University) for his help.
Appendix A. Supplementary dataSupplementary material related to this article can be found, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2019.07.027.
| [1] |
S.O. McDonnell, M.J. Hall, D.F. O'Shea, et al., J. Am. Chem. Soc. 127 (2005) 16360-16361. DOI:10.1021/ja0553497 |
| [2] |
H. Lu, J. Mack, Z. Shen, et al., Chem. Soc. Rev. 43 (2014) 4778-4823. DOI:10.1039/C4CS00030G |
| [3] |
S. Long, Q. Qiao, Z. Xu, et al., Chin. Chem. Lett. 30 (2019) 573-576. DOI:10.1016/j.cclet.2018.11.031 |
| [4] |
W. Liu, J. Yao, C. Zhan, Chin. Chem. Lett. 28 (2017) 875-880. DOI:10.1016/j.cclet.2017.01.013 |
| [5] |
A. Xie, Z. Pan, D. Sun, et al., Chin. Chem. Lett. 30 (2019) 875-880. DOI:10.1016/j.cclet.2019.03.016 |
| [6] |
Y. Cakmak, S. Kolemen, E.U. Akkaya, et al., Angew. Chem. Int. Ed. 50 (2011) 11937-11941. DOI:10.1002/anie.201105736 |
| [7] |
W. Pang, X.F. Zhang, L. Jiao, et al., Chem. Commun. 48 (2012) 5437-5439. DOI:10.1039/c2cc30915g |
| [8] |
M.T. Whited, N.M. Patel, M.E. Thompson, Chem. Commun. 48 (2012) 284-286. DOI:10.1039/C1CC12260F |
| [9] |
G.M. Fischer, A.R. Ehlers, E. Daltrozzo, et al., Angew. Chem. Int. Ed. 46 (2007) 3750-3753. DOI:10.1002/anie.200604763 |
| [10] |
I.S. Tamgho, A. Hasheminasab, C.J. Ziegler, et al., J. Am. Chem. Soc. 136 (2014) 5623-5625. DOI:10.1021/ja502477a |
| [11] |
C. Yu, L. Jiao, E. Hao, et al., Org. Lett. 16 (2014) 1952-1955. DOI:10.1021/ol500507f |
| [12] |
J. Wang, Q. Wu, L. Jiao, et al., J. Org. Chem. 81 (2016) 11316-11323. DOI:10.1021/acs.joc.6b02291 |
| [13] |
X.D. Jiang, Y. Su, S. Yue, et al., RSC Adv. 5 (2015) 16735-16739. DOI:10.1039/C4RA15914D |
| [14] |
C. Yu, L. Jiao, E. Hao, et al., Org. Lett. 20 (2018) 4462-4466. DOI:10.1021/acs.orglett.8b01752 |
| [15] |
X.D. Jiang, S. Li, B. Le Guennic, et al., Phys. Chem. Chem. Phys. 18 (2016) 32686-32690. DOI:10.1039/C6CP05705E |
| [16] |
X. Jiang, T. Zhang, L. Xiao, et al., Chin. Chem. Lett. 30 (2019) 1055-1058. DOI:10.1016/j.cclet.2019.02.016 |
| [17] |
X. Jiang, J. Zhao, D. Xi, et al., Chem. Eur. J. 21 (2015) 6079-6082. DOI:10.1002/chem.201406535 |
| [18] |
X. Jiang, X. Liu, T. Fang, et al., Dyes Pigm. 146 (2017) 438-444. DOI:10.1016/j.dyepig.2017.07.038 |
| [19] |
X. Jiang, J. Guan, J. zhao, et al., Dyes Pigm. 136 (2017) 619-626. DOI:10.1016/j.dyepig.2016.09.019 |
| [20] |
T. Fang, X. Jiang, C. Sun, et al., Sens. Actuators B Chem. 290 (2019) 551-557. DOI:10.1016/j.snb.2019.03.141 |
| [21] |
Q. Zheng, G. Xu, P.N. Prasad, Chem. Eur. J. 14 (2008) 5812-5819. DOI:10.1002/chem.200800309 |
| [22] |
D. Wang, Y. Shiraishi, T. Hirai, Tetrahedron Lett. 51 (2010) 2545-2549. DOI:10.1016/j.tetlet.2010.03.013 |
| [23] |
X. Liu, Y. Li, X. Song, et al., Chem. Commun. 54 (2018) 1509-1512. DOI:10.1039/C7CC08154E |
| [24] |
Topics in fluorescence spectroscopy, in: B. Valeur, J.R. Lakowicz (Eds.), Probe Design and Chemical Sensing, 4 Plenum, New York, 1994, pp. 21.
|
| [25] |
S.O. McDonnell, D.F. O'Shea, Org. Lett. 8 (2006) 3493-3496. DOI:10.1021/ol061171x |
| [26] |
K. Rurack, M. Kollmannsberger, J. Daub, Angew. Chem. Int. Ed. 40 (2001) 385-387. DOI:10.1002/1521-3773(20010119)40:2<385::AID-ANIE385>3.0.CO;2-F |
 2019, Vol. 30
2019, Vol. 30