b Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Department of Chemistry, Fudan University, Shanghai 200438, China
In recent decades, supramolecular gels have gained considerable attentions due to their diverse applications in the fields ranging from chemistry, physics, biology to material sciences [1-5]. Among them, the less-studied molecular metallogels are considered as one of the most fascinating "smart" soft materials [6, 7], probably due to integrating the specific characteristics of metals into the gels. Along with other non-covalent interactions (such as van der Waals, π-π stacking and hydrogen bonding interactions), incorporation of metal provides subtle control of molecular assembly, gel morphology and stimuli responsive properties by tuning the metal-metal interactions [8-11]. Furthermore, suitable guest molecules might also assist or hinder these weak interactions to benefit metallogels formation or collapse, which also provides a new plausible approach for visual recognition of the guest molecules [12-14].
Due to its high nitrogen content (66%) and low price, melamine has been selected and illegally added to increase the protein reading level of dairy products [15-17]. However, melamine can induce renal disorder and even death, especially, for infants [18-21]. Consequently, a variety of expensive instruments, including liquid chromatography-mass spectrometry (LC–MS) [22], gas chromatography-mass spectrometry (GC–MS) [23], low-temperature plasma-mass spectrometry (LTP-MS) [24], surface-enhanced Raman scattering (SERS) [25], and enzyme-linked immunosorbent assay (ELISA) [26], have been applied for melamine detection. Although high sensitivity, high accuracy and low detection limits are usually observed, the detectable samples have to be tediously pre-processed and extracted before the analysis by using these expensive and complicated instruments [27-29], which is hardly suitable for the real-time and on-site direct detection of melamine in dairy products, especially for nonspecialists.
In consideration of the reversibility, specificity, directionality of hydrogen bonding between melamine and thymine or its derivatives [30], such complementary triple N—H…O and N—H…N interactions have been regarded as a key issue for several reported melamine detection via molecular assembly [31]. However, to the best our knowledge, there is only one example on visual detection of melamine via molecular gel formation with peptide-based cyanuric acid derivative [32]. No metallo-hydrogel formation has been reported for this purpose. Following our recent research interests in metallogel fabrication, characterization and their potential application in visual discrimination of isomers [33], homologues [34], and bioactive molecules [35], we would like to extend the study to develop a simple, straightforward and on-site protocol for melamine detection of dairy samples via selective metallo-hydrogel formation.
In order to realize our hypothesis, a series of novel unsymmetrical pincer zinc complexes 1a and 1b (Scheme 1), containing a thymine fragment and a quaternary ammonium tail with different length of linkers, were therefore designed and synthesized. In light of the low solubility of pincer zinc complexes in water, the ammonium tail was introduced to increase the complex solubility. The synthetic route was described in Scheme 1. By condensation of 1-(pyridin-2-yl)ethanone, 4-hydroxyl-benzaldehyde and aqueous ammonium in ethanol under basic conditions, teypridine 2 was synthesized, which is readily converted to terpyridine-substituted alkyl bromides 3a and 3b with different alkyl lengths (n = 3 and 6). After amination reaction with thymine or triethylamine, thymine derivatives (4a and 4b) and quaternary ammonium bromides (5a and 5b) were then obtained. After coordination of zinc acetate, quaternary ammonium bromides 5a and 5b were readily converted into terpyridine zinc complexes 6a and 6b in methanol at room temperature. Finally, by simply mixing thymine derivatives 4a and 4b and terpyridine zinc complexes 6a and 6b in DMF, unsymmetric pincer zinc complexes 1a and 1b with two different terminals were obtained, which could be further purified by simply crystallization from DMF and ethyl acetate.

|
Download:
|
| Scheme 1. Synthesis of unsymmetrical pincer zinc complexes 1a-c. | |
Unsymmetrical pincer zinc complexes 1a and 1b are soluble in water at relatively high concentration. For example, 60 mg 1b is readily dissolved in 1 mL water under ambient conditions (6 wt%), higher amount of 1b could also be dissolved by gentle heating. To our delight, when a drop of saturated aqueous solution of melamine was added into 1 wt% of 1b aqueous solution, after heating and cooling, a transparent metallo-hydrogel was readily formed, which may be considered as a new approach for visual discrimination of melamine via metallhydorgel formation. Subsequently, a series of gelation tests with different ratios of complex 1b and melamine (from 3:1 to 1:3) at different concentration (1–6 wt%) were carried out (Table 1). Pleasingly, even at low gelation concentrations (0.5–1 wt%), gel formation could be still observed in some cases, though the gel was relatively weak (WG). The gel-sol phase-transition temperature (Tg) was determined by the "dropping-ball method" (Table 1). Higher stability and Tg were observed with the gel containing more amount of pincer complex 1b and melamine. Unexpectedly, the higher amount of melamine induced higher thermostability and Tg. Similar outcomes were also observed for pincer complex 1a, with slightly worse stability of the corresponding metallo-hydrogels, probably due to the shorter alkyl chains. Although no gel formation was observed with normal organic solvents, a broad range of alcoholic solvents are suitable for gelation, and the resulting metallogels exhibited higher Tg values than those in water (Table S1 in Supporting information).
|
|
Table 1 Gelation test with pincer complex 1b and melamine (M) in different concentrations and ratios in water.a |
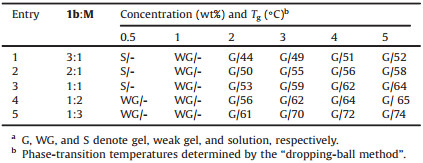
The morphologies of xerogel networks obtained from complex 1a or 1b and melamine with the corresponding solvents were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Typical SEM images are presented in Figs. 1a and b. At 2 wt% and 1:3 ratio of 1a and M, wavy-hair-like fibers with approximately 2–5 μm long and up to 30 nm wide were observed in the metallo-hydrogel 1a (Fig. 1a). In contrast, the image of 3 wt% metallo-hydrogel 1b at the same ratio was slightly different and revealed long (up to 10 μm) and thin (ca. 20 nm wide) fibers (Fig. 1b). The same morphologies were observed in the TEM images (Figs. 1c and d). These long and thin gel fibers were also found in the metallo-gels 1b/1-butanol and 1b/ cyclohexanol (Figs. S44 and S47 in Supporting information). Plates morphologies were observed for the metallo-gels 1b in 1-pentanol and cyclopentanol (Figs. S45 and S46 in Supporting information).
|
Download:
|
| Fig. 1. SEM and TEM images of metallo-hydrogels of (a, c) 1a + M (1:3 at 2 wt%), and (b, d) 1b + M (1:3 at 3 wt%). | |
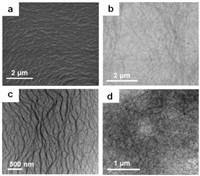
In order to explore the possible driving forces for metallohydrogel formation and plausible mechanism of visual recognition, a series of experiments were carried out. Initially, temperaturedependent 1H NMR spectroscopy studies of metallo-hydrogel 1b in D2O (1:3 at 2 wt%) were involved. To our surprise, in contrast to the 1H NMR spectra of 1b at 75 ℃, there are less numbered but clearly broad signals at 25 ℃ (Fig. 2), indicating strong but complementary aggregation of pincer complex 1b and melamine at the gel state under the ambient conditions. Upon warming to 75 ℃ by a step of 10 ℃, the signals of the gel complex steadily sharpened. Compared with initial broad peaks at 25 ℃, the N—H signal of thymine fragment (HT) and O—H signal of CH2 on the Et3N+C6 chain (HM) were steadily and obviously increased and downshifted (from 6.44 and 3.83 ppm to 7.11 and 4.45 ppm, respectively) along with the test temperature rising to 75 ℃. These outcomes clearly indicated that the complementary triple hydrogen bonds between thymine fragment melamine were one of the main interactions responsible for the gelation process. Besides, the Hβ at the β-position of the middle pyridine was also downshifted (from 8.28 ppm to 8.72 ppm), which clearly suggested that π-π stacking between pyridines also contribute to the aggregation.
|
Download:
|
| Fig. 2. Temperature-dependent 1H NMR spectra of metallo-hydrogel formed by complex 1b and melamine (1:3 at 2 wt%) in D2O. | |
With these clues in our minds, control experiments involving pincer zinc complex 1c (methyl protected analogue of 1b, Scheme 1), triaminobenzene (a melamine analogue), the possible milk ingredients (like amino acids, illegal/legal additives including sugars, vitamins and urea) were carried out. When pincer complex 1c or triaminobenzene was applied instead, under the standard gelation conditions (1:3 ratio at 2 wt%), no gelation was found in both cases (Fig. S60 in Supporting information). The results further confirmed that the importance of triple hydrogen bonds during assembly. Subsequently, when urea, lactose, glucose, glutamic, histidine and cysteine were used instead of melamine under the standard gelation conditions, no gelation was observed either (Fig. 3), though all these selected substances can form weak hydrogen bonds with thymine fragment. Hence, the visual recognition of melamine is highly selective via selective metallo-hydrogel formation.

|
Download:
|
| Fig. 3. Control experiments with various illegal/legal additives in milk for gelation test (6 mg additives with 3 wt% of complex 1b). | |
We believe the direction and robustness of these complementary triple hydrogen bonds (Fig. 4c) will benefit molecular assembly to form a π-plate between melamine and thymine fragment, which can further yield fiber unit through π-π stacking interactions between the π-plates (Fig. 4b, side view). After twining, elongating and extending of the fiber units, long and thin fibers are therefore formed, which can trap the water molecules leading to formation of metallo-hydrogels (Fig. 4a).

|
Download:
|
| Fig. 4. Plausible assembly process of metallo-hydrogel prepared from pincer complex 1b and melamine: a) metallo-hydrogel and gel fibers trapped water, b) fiber units formed via π-π stacking; and c) complementary triple hydrogen-bonding | |
Having the plausible aggregation and sensing mechanism in hand, we would like to test the visual recognition ability of the pincer complex 1b to trace amount of melamine. After heating a vial containing 30 mg pincer complex 1b in 0.5 mL water, no gel formation but a clear solution (A) was observed after heating and cooling process (Fig. 5a). When a drop of saturated aqueous melamine solution was added into the clear solution (A), a transparent gel was then formed after the standard heating– cooling process within 5 min (Fig. 5a). Subsequently, a series of aqueous melamine solution with different concentrations (1– 1000 ppm) was added (0.5 mL) into a vial containing 30 mg pincer complex 1b for visual discrimination. When the final concentration of melamine in the vial was greater than 10 ppm, gel formations were observed in all corresponding vials within a few minutes. Therefore, this protocol is promising for visual detection of melamine without usage of expensive and professional instruments.

|
Download:
|
| Fig. 5. Visual recognition of melamine by using a) a vial containing 6 wt% aqueous solution of pincer complex 1b (solution A) with or without 10 ppm melamine in water, b) 6 wt% raw milk solution of pincer complex 1b (solution B) with or without 10 ppm melamine. | |
Be aware of highly selective visual recognition of trace amount of melamine in aqueous samples and no impacts of milk ingredients, legal and illegal additives, we would like to investigate the possibility of using this protocol to detect trace amount of melamine added in raw milk without any pretreatment. To our delight, when 0.5 mL of the milk sample containing melamine (10 ppm) was added into the vial containing 30 mg pincer complex 1b, a white turbid gel (6 wt%) was formed within 10 min after typical heating and cooling process (Fig. 5b). On the contrary, no gelation was observed for the milk sample without melamine under the otherwise identical reaction conditions (Fig. 5b). The gel formation is repeatable for several heating and cooling cycles, which further confirmed the visual discrimination ability of 1b to melamine in raw milk. Compared with other known melamine detection approaches and even instrumental analysis involving GC–MS, HPLC-MS and LTP-MS, our newly developed visual recognition of melamine via selective metallo-hydrogel formation is much cheaper, straightforward and convenient. Even without any pretreatment, a sensitivity (10 ppm) comparable to instrumental analysis is achieved.
In conclusion, a cheap, straightforward and convenient approach for visual recognition of melamine via selective metallo-hydrogel formation has been established, even for raw milk samples without any pretreatment. The strong triple hydrogen-bonding interactions between melamine and thymine terminal of the unsymmetrical pincer zinc complex 1b and additional π-π stacking may be responsible for the selective metallo-hydrogel formation, which have been confirmed by temperature-dependent 1H NMR studies and related control experiments. Remarkably, even with raw milk samples, a comparable limit of detection to instrumental analysis is achieved, highlighting its potential application for quick practical on-site melamine-detection.
AcknowledgmentsFinancial support from the State General Administration of the People's Republic of China for Quality Supervision and Inspection and Quarantine (No. 2016QK122), Shanghai Institute of Quality Inspection and Technical Research, the National Natural Science Foundation of China (Nos. 21572036 and 21861132002), and the Department of Chemistry, Fudan University is gratefully acknowledged.
Appendix A. Supplementary dataSupplementary material related to this article canbe found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.07.025.
| [1] |
W.G. Miao, S. Wang, M.H. Liu, Adv. Funct. Mater. 27 (2017) 1701368. DOI:10.1002/adfm.201701368 |
| [2] |
K.Q. Liu, S. Gao, Z. Zheng, et al., Adv. Mater. 31 (2019) 1808254. DOI:10.1002/adma.201808254 |
| [3] |
J. Cheng, S. Feng, S. Han, et al., ACS Nano 10 (2016) 9957-9973. DOI:10.1021/acsnano.6b04124 |
| [4] |
C. Wang, Q. Chen, F. Sun, et al., J. Am. Chem. Soc. 132 (2010) 3092-3096. DOI:10.1021/ja910721s |
| [5] |
J.Y. Zhang, L.H. Zeng, J. Feng, Chin. Chem. Lett. 28 (2017) 168-183. DOI:10.1016/j.cclet.2016.07.015 |
| [6] |
Y. Sun, S. Li, Z.X. Zhou, et al., J. Am. Chem. Soc. 9 (2018) 3257-3263. |
| [7] |
Y. Sun, S. Li, Z.X. Zhou, et al., J. Am. Chem. Soc. 140 (2018) 3257-3263. DOI:10.1021/jacs.7b10769 |
| [8] |
H. Yin, F. Dumur, Y.M. Niu, et al., ACS Appl. Mater. Interfaces 9 (2017) 33220-33228. DOI:10.1021/acsami.7b06634 |
| [9] |
Y. Meng, J. Jiang, M.H. Liu, Nanoscale 9 (2017) 7199-7206. DOI:10.1039/C7NR02126G |
| [10] |
W. Zhang, Z.G. Xie, Chin. Chem. Lett. 27 (2016) 77-80. DOI:10.1016/j.cclet.2015.09.009 |
| [11] |
J.Y. Fan, X.M. Chang, M.X. He, et al., ACS Appl. Mater. Interfaces 828 (2016) 18584-18592. |
| [12] |
W.G. Miao, L. Zhang, X.F. Wang, et al., Langmuir 29 (2013) 5435-5442. DOI:10.1021/la400562f |
| [13] |
Q. Lia, C. Liu, J. Wen, et al., Chin. Chem. Lett. 28 (2017) 1857-1874. DOI:10.1016/j.cclet.2017.05.007 |
| [14] |
C. Wang, D.Q. Zhang, D.B. Zhu, Langmuir. 23 (2007) 1478-1482. DOI:10.1021/la062621x |
| [15] |
L. Tang, S. Mo, S.G. Liu, J. Agric. Food Chem. 66 (2018) 2174-2179. DOI:10.1021/acs.jafc.7b05245 |
| [16] |
S. Xu, G.Y. Lin, W. Zhao, et al., ACS Appl. Mater. Interfaces 10 (2018) 24850-24859. DOI:10.1021/acsami.8b08558 |
| [17] |
W.J. Qi, D. Wu, J. Ling, et al., Chem. Commun. 46 (2010) 4893-4895. DOI:10.1039/c0cc00886a |
| [18] |
C. Hazra, V.N.K.B. Adusumalli, V. Mahalingam, ACS Appl. Mater. Interfaces 6 (2014) 7833-7839. DOI:10.1021/am5011089 |
| [19] |
Y. Wang, Q.Q. Sun, L.L. Zhu, et al., Chem. Commun. 51 (2015) 7958-7961. DOI:10.1039/C5CC01660F |
| [20] |
J. Lee, E.J. Jeong, J. Kim, Chem. Commun. 47 (2011) 358-360. DOI:10.1039/C0CC02183K |
| [21] |
C.X. Niu, Q.L. Liu, Z.H. Shang, et al., Nanoscale 7 (2015) 8457-8465. DOI:10.1039/C5NR00554J |
| [22] |
A.M.R. Mondal, A. Desmarchelier, E. Konings, et al., J. Agric. Food Chem. 58 (2010) 11574-11579. DOI:10.1021/jf102900k |
| [23] |
Y.L. Wong, C.S. Mok, Anal. Methods 5 (2013) 2305-2314. DOI:10.1039/c3ay26459a |
| [24] |
G.M. Huang, W. Xu, M.A. Visbal-Onufrak, et al., Analyst 135 (2010) 705-711. DOI:10.1039/B923427F |
| [25] |
Q.Q. Yang, F.H. Liang, D. Wang, et al., Anal. Methods 6 (2014) 8388-8395. DOI:10.1039/C4AY00965G |
| [26] |
T.H. Tsoi, W.T. Wong, Anal. Methods 7 (2015) 5989-5995. DOI:10.1039/C5AY00648A |
| [27] |
Y. Li, J.Y. Xu, C.Y. Sun, RSC Adv. 5 (2015) 1125-1147. DOI:10.1039/C4RA13080D |
| [28] |
L.L. Li, G.H. Wu, T. Hong, et al., ACS Appl. Mater. Interfaces 6 (2014) 2858-2864. DOI:10.1021/am405305r |
| [29] |
M. Farrokhnia, S. Karimi, S. Askarian, ACS Sustainable Chem. Eng. 7 (2019) 6672-6684. DOI:10.1021/acssuschemeng.8b05785 |
| [30] |
K.L. Ai, Y.L. Liu, L.H. Lu, J. Am. Chem. Soc. 131 (2009) 9496-9497. DOI:10.1021/ja9037017 |
| [31] |
J.J. Du, Z.K. Wang, X.J. Peng, et al., Ind. Eng. Chem. Res. 54 (2015) 12011-12016. DOI:10.1021/acs.iecr.5b02399 |
| [32] |
J.W. Zhang, C.W. Ou, Y. Shi, et al., Chem. Commun. 50 (2014) 12873-12876. DOI:10.1039/C4CC05826G |
| [33] |
T. Tu, W.W. Fang, X.L. Bao, et al., Angew. Chem. Int. Ed. 50 (2011) 6601-6605. DOI:10.1002/anie.201100620 |
| [34] |
W.W. Fang, X.Y. Liu, Z.W. Lu, et al., Chem. Commun. 50 (2014) 3313-3316. DOI:10.1039/C3CC49402K |
| [35] |
W.W. Fang, C. Liu, F.B. Yu, et al., ACS Appl. Mater. Interfaces 8 (2016) 20583-20590. DOI:10.1021/acsami.6b05804 |
 2019, Vol. 30
2019, Vol. 30