b Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha 410114, China;
c School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
d School of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China
Green chemistry aims at the employment of renewable environment-benign materials as well as minimizing the generation of chemical waste, and it is of particular significance to develop sustainable synthetic protocols for that purpose. For most chemical synthesis, a large amount of petroleum solvents are required to facilitate the reaction process, which not only consumes many non-renewable fossil resources but also results in serious environmental issue. Therefore, a lot of effort has been paid to substitute conventional hazardous organic solvents with eco-friendly ones to conserve non-renewable fossil fuels [1]. Typical examples include biomass-derived solvents [2], ionic liquid [3], and water [4], which are either abundant in nature or not limited by petroleum resources. Of the developed biomass-derived solvents, 2-methyltetrahydrofuran (2-MeTHF) is a rising star which has drawn increasing attention, because it can be obtained from biomass feedstocks as a safe and innocuous solvent, allowing an exposure in human up to 6.2 mg per day, and it is down gradable when subjected to environmental factors like ambient air or sunlight. Therefore, being as a suitable green solvent, it is in line with the 3rd (less hazardous chemical synthesis), 5th (safer solvents and auxiliaries), 7th (use of renewable feedstocks) and the 10th (design for degradation) principles of green chemistry, and during the past years a lot of synthetic reactions have been executed by using 2-MeTHF as the reaction medium [5].
Sulfur-containing compounds have received considerable attention from organic and medicinal chemists due to their versatility in organic synthetic procedures and their valuable biological activities [6]. Among them, the S-thiocarbamates is an essential ingredient in many pharmaceuticals and agrochemicals, and they have also been employed as important building block in organic synthesis [7]. During the past few decades, numerous efforts have been made to prepare such motifs. However, most synthetic protocols have one or more disadvantages, such as the usage of highly harmful chemical reactants (monoxide, phosgene, isocyanates and thiols), harsh reaction conditions and narrow scopes [8]. Recently, Wei and coworkers pioneered the molecular iodine-catalyzed synthesis of thiocarbamates through multicomponent reaction [9] of aryl sodium sulfinates, isonitriles and water in the presence of dimethyl phosphite (2 equiv.) as the reductant in ethyl acetate (EtOAc) [10]. Very recently, our group developed the I2-catalyzed synthesis of thiocarbamates with sulfonyl chlorides using triphenylphosphine (PPh3, 2 equiv.) as the reductant in N-methylpyrrolidone (NMP) [11]. However, these methods employed a lot of petroleum based volatile organic chemicals as the reaction medium. Moreover, they still require a large amount of toxic phosphorus-containing compounds as the reductants, thus plenty of harmful chemical waste is inevitably produced and the tedious organic solvent exaction is inevitable.
As a part of our research program in green chemistry [12], herein, we wish to report a simple and eco-friendly protocol for the clean preparation of S-thiocarbamates using 2-MeTHF as the solvent (Scheme 1c). The remarkable achievements of our developed catalytic reaction are: (1) neither petroleum-solvent nor harmful phosphorus-containing reductant is required; (2) in situ generated hydroxide instead of external water serves as the reductant; (3) water and nitrogen are generated as clean sideproducts, thus the tedious extraction procedure can be avoided.

|
Download:
|
| Scheme 1. Synthesis of S-thiocarbamates. | |
Benzenesulfonylhydrazine (1a) with tert-butylisonitrile (2a) was chosen as a model reaction for the condition optimization. By employing 10 mol% KI as a catalyst under an air atmosphere at 90 ℃, a 64% NMR yield of S-phenyl tert-butylcarbamothioate (3aa) was obtained after 4 h when 2-MeTHF was applied as a reaction medium (Table 1, entry 1). To increase the reaction efficiency, various other catalysts (entries 2–7) were investigated and the results showed that NaI was a superior catalyst affording a 69% yield (entry 2). The exploration on the suitable loading of NaI (entries 8–10) revealed that 20 mol% NaI (entry 8) was an optimal amount for this transformation. No further improvement was observed when other solvents, such as THF, DMF, EtOAc, 1, 4- dioxane, DMSO, N-methyl pyrrolidone (NMP) and water (entries 11–17) was used instead of 2-MeTHF. No improvement in the reaction efficiency was observed when surfactant (10 wt% sodium dodecyl sulfate) was employed (entry 18). Reaction temperature assessment (entries 19 and 20) showed that 90 ℃ was an appropriate reaction temperature for the reaction. Changing the air atmosphere to nitrogen atmosphere elevated the reaction efficiency and the desired 3aa was generated in an 85% NMR yield (entry 21). Performing the reaction under oxygen atmosphere did not provide improved yield of the desired product (entry 22). Control experiment revealed that the NaI catalyst was essential for the novel eco-friendly thiocarbamation reaction (entries 23).
|
|
Table 1 Optimization of reaction conditions.a |
With the above optimized conditions, we next investigated the reaction scope of the present reaction. As shown in Scheme 2, various aryl sulfonylhydrazines bearing either electron-rich or electron-poor groups employed underwent the thiocarbamation reaction efficiently to deliver the expected products (3aa–3pa) in good to excellent yields. Moreover, a series of valuable functional groups are well compatible, such as alkyl (3ba–3ca, 3ka and 3ma), alkoxy (3 da), free hydroxyl (3ea), halide (3fa-3ha, 3la and 3na), trifluoromethyl (3ia), and amide (3ja) group. Sterically hindered arylsulfonylhydrazines such as 2-methyl (1m) and 2-chlorobenzenesulfonohydrazide (1n) also survived the standard reaction conditions to form the corresponding products in excellent yields (3ma and 3na). The polysubstituted phenyl-(1o and 1p), naphthyl-(1q) and heteroaryl-sulfonohydrazide (1r and 1s) were suitable as substrates in the current transformation and produced the desired products (3oa–3sa) in good yields. Additionally, alkyl sulfonohydrazides (1t and 1u) were also applied as good substrates and generated the expected products 3ta and 3ua in 72% and 68% yield, respectively. Finally, a series of isonitriles were used in the reactions. Not only tert-butyl isonitriles, but also secondary and primary isonitriles could be converted into to the corresponding carbamothioates (3bb–3bf). Functional-groups, including benzyl, ester and sulfonyl, were tolerent of the developed reaction (see Supporting information).

|
Download:
|
| Scheme 2. Substrate scope. All reactions were carried out in a seal tube in the presence of 1 (0.2 mmol), 2 (0.26 mmol), NaI (0.04 mmol), 2-MeTHF (1 mL), 90 ℃, nitrogen atmosphere, 4 h. b 100 ℃, 5 h. c I2 instead of NaI, 1, 4-dioxane instead of 2-MeTHF, 100 ℃, 6 h. | |
As an easy-to-operate and effective protocol, the gram-scale of carbamothioation reaction has practical significance for the synthesis of various secondary S-thiocarbamates in academic laboratory and industry. When 7 mmol of benzenesulfonylhydrazine (1a) was reacted with tert-butylisonitrile under the standard conditions, the product 3aa could be obtained with a good yield of 76%, demonstrating a great potential application in pharmaceutical synthesis (Scheme 3). However, decreasing the loading of 2-MeTHF led to a decrease in the yield of 3aa.

|
Download:
|
| Scheme 3. Large-scale synthesis of 3aa. | |
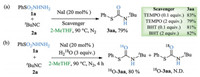
To elucidate the reaction mechanism, some control experiments were carried out as shown in Scheme 4. When 0.1 or 2 equiv. of TEMPO or BHT were added to the reaction mixture under the standard reaction conditions (Scheme 4a) the yield of 3aa was not notably influenced, which revealed that the present carbamothioation reaction may not proceed via a free-radical process but probably in an ionic pathway. Performing the reaction with three equiv. of H218O, no 18O-3aa was observed by GC–MS, suggesting that the oxygen atom of carbamothioate did not originate from water (Scheme 4).
|
Download:
|
| Scheme 4. Control experiments. | |
Based on the aforementioned experimental results and the related studies [10, 11], a proposed reaction pathway was illustrated in Scheme 5. First, sulfonyl hydrazide 1 with catalytic amount of iodide resulted in the formation of intermediate R-S-I (A) with the release of N2, H2O and hydroxide (OH-) [13]. Subsequently, the reaction of isonitrile 2 with intermediate A generated the intermediate B, which was attacked by OH- to produce carbonimidothioate intermediate C and regenerated the iodide ion to complete the catalytic cycle. Finally, the intermediate C upon tautomerization (i.e., migration of proton) formed the target product 3.

|
Download:
|
| Scheme 5. Plausible reaction mechanism. | |
In conclusion, a simple and clean protocol for the synthesis of S-thiocarbamates has been established. The usage of in situ generated hydroxide both as an oxygen source and hydrogen source as well as 2-methyltetrahydrofuran as a green reaction medium, and the avoidance of phosphorus-containing reductant have made the present method atom-economic and environmentally friendly. A series of alkyl and (hetero)aryl S-thiocarbamates were prepared in good to excellent yields. In the viewpoint of green and sustainable chemistry, this current protocol is very attractive compared with the previous reports, owing to the avoidance of non-renewable petroleum-based solvents, clean water and nitrogen as the side-products, easy operation and simple work-up (without extraction). This research could open the possibilities in the future application of biomass resource and provide new approach for the utilization of side-product instead of external additives.
AcknowledgmentWe are grateful for financial support from the Hunan Provincial Natural Science Foundation of China (Nos. 2019JJ50193 and 2019JJ20008).
Appendix A. Supplementary dataSupplementary material related to this article canbe found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.06.052.
| [1] |
Y. Gu, F. Jerome, Chem. Soc. Rev. 42 (2013) 9550-9570. DOI:10.1039/c3cs60241a |
| [2] |
(a) J. Yang, J.N. Tan, Y. Gu, Green Chem. 14 (2012) 3304-3317; (b) R. Bai, P. Liu, J. Yang, C. Liu, Y. Gu, ACS Sustainable Chem. Eng. 3 (2015) 1292-1297; (c) S.G. Zhang, Z.B. Xie, L.S. Liu, M. Liang, Z.G. Le, Chin. Chem. Lett. 28 (2017) 101-104; (d) G. Gao, P. Wang, P. Liu, et al., Chin. J. Org. Chem. 38 (2018) 846-854; (e) L. Li, Q. Chen, X. Xiong, et al., Chin. Chem. Lett. 29 (2018) 1893-1896. |
| [3] |
(a) H. Li, C. Liu, Y. Zhang, et al., Org. Lett. 17 (2015) 932-935; (b) D. Cao, Y. Zhang, C. Liu, et al., Org. Lett. 18 (2016) 2000-2003; (c) B. Lai, R. Bai, Y. Gu, ACS Sustainable Chem. Eng. 6 (2018) 17076-17086; (d) G.P. Yang, X. Wu, B. Yu, C. Hu, ACS Sustainable Chem. Eng. 7 (2019) 3727-3732; (e) D.Q. Dong, W.J. Chen, Y. Yang, X. Gao, Z.L. Wang, Chem. Select 4 (2019) 2480-2483. |
| [4] |
(a) R. Mohebat, A. Yazdani-Elah-Abadi, M. Malek-Taher, N. Hazeri, Chin. Chem. Lett. 28 (2017) 943-948; (b) L.Y. Xie, Y. Duan, L.H. Lu, et al., ACS Sustainable Chem. Eng. 5 (2017) 10407-10412; (c) Q.Q. Xuan, Y.H. Wei, Q.L. Song, Chin. Chem. Lett. 28 (2017) 1163-1166; (d) D.Q. Dong, S.H. Hao, H. Zhang, Z.L. Wang, Chin. Chem. Lett. 28 (2017) 1597-1599; (e) M. Zhang, Q.Y. Fu, G. Gao, et al., ACS Sustainable Chem. Eng. 5 (2017) 6175-6182; (f) H. Cui, W. Wei, D. Yang, et al., Green Chem. 19 (2017) 3520-3524; (g) K. Sun, X.L. Chen, S.J. Li, et al., J. Org. Chem. 83 (2018) 14419-14430; (h) P. Bao, L. Wang, Q. Liu, et al., Tetrahedron Lett. 60 (2019) 214-218; (i) Y. Liu, L. Chen, Z. Wang, et al., J. Org. Chem. 84 (2019) 204-215; (j) Y. Zheng, M. Liu, G. Qiu, W. Xie, J. Wu, Tetrahedron 75 (2019) 1663-1668; (k) Y.H. Wang, B. Ouyang, G. Qiu, H. Zhou, J.B. Liu, Org. Biomol. Chem. 17 (2019) 4335-4341; (l) K. Sun, S.J. Li, X.L. Chen, et al., Chem. Commun. 55 (2019) 2861-2864; (m) J. Li, W. Tang, D. Ren, J. Xu, Z. Yang, Green Chem. 21 (2019) 2088-2094; (n) Y.C. Wang, R.X. Wang, G. Qiu, et al., Org. Chem. Front. 6 (2019) 2471-2479. |
| [5] |
A.D. Mamuye, S. Monticelli, L. Castoldi, W. Holzer, V. Pace, Green Chem. 17 (2015) 4194-4197. DOI:10.1039/C5GC01002K |
| [6] |
(a) W. Xie, S. Xie, Y. Zhou, et al., Eur. J. Med. Chem. 81 (2014) 22-27; (b) X. Gong, J. Chen, X. Li, W. Xie, J. Wu, Chem. -Asian J. 13 (2018) 2543-2548; (c) W. Xie, Y. Wu, J. Zhang, et al., Eur. J. Med. Chem. 145 (2018) 35-40; (d) L. Xue, Y. Liu, W. Qin, H. Yan, Chin. Chem. Lett. 29 (2018) 1215-1218; (e) Q. Liang, Y. Zhang, M. Zeng, et al., Toxicol. Res. 7 (2018) 521-528; (f) G. Xiao, H. Min, Z. Zheng, G. Deng, Y. Liang, Chin. Chem. Lett. 29 (2018) 1363-1366; (g) Q. Liu, L. Wang, H. Yue, et al., Green Chem. 21 (2019) 1609-1603; (h) H. Jiang, X. Tang, Z. Xu, et al., Org. Biomol. Chem. 17 (2019) 2715-2720; (i) J. Xu, W. Huang, R. Bai, et al., Green Chem. 21 (2019) 2061-2069; (j) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. (2019), doi: http://dx.doi.org/10.1016/j.cclet.2019.05.041; (k) D.Q. Dong, L.X. Li, G.H. Li, Q. Deng, Z.L. Wang, Chin. J. Catal. 40 (2019) 1494-1498; (l) Y. Liu, X.L. Chen, K. Sun, et al., Org. Lett. 21 (2019) 4019-4024; (m) A. El-Harairy, Y. Qi, B. Lai, et al., Adv. Synth. Catal. 361 (2019) 3342-3350; (n) C. Zhu, D. Wei, Y. Wu, et al., J. Alloys Compd. 778 (2019) 731-740; (o) F.L. Zeng, X.L. Chen, S.Q. He, et al., Org. Chem. Front. 6 (2019) 1476-1480; (p) X.M. Xu, D.M. Chen, Z.L. Wang, Chin. Chem. Lett. (2019), doi: http://dx.doi.org/10.1016/j.cclet.2019.05.048; (q) X. Gong, G. Li, Z. Gan, et al., Asian J. Org. Chem. 8 (2019) 1472-1478. |
| [7] |
(a) T. Mizuno, I. Nishiguchi, T. Okushi, T. Hirashima, Tetrahedron Lett. 32 (1991) 6867-6868; (b) A.W. Erian, S.M. Sherif, Tetrahedron 55 (1999) 7957-8024; (c) J. Zhu, B. Xu, J. Yu, et al., Org. Biomol. Chem. 16 (2018) 5999-6005. |
| [8] |
(a) R.S. Pathare, V. Patil, H. Kaur, et al., Org. Biomol. Chem. 16 (2018) 8263-8266; (b) W.H. Bao, C. Wu, J.T. Wang, et al., Org. Biomol. Chem. 16 (2018) 8403-8407; (c) W. Wei, P. Bao, H. Yue, et al., Org. Lett. 20 (2018) 5291-5295; (d) X. Gong, X. Li, W. Xie, J. Wu, S. Ye, Org. Chem. Front. 6 (2019) 1863-1867. |
| [9] |
(a) G. Li, Z. Gan, K. Kong, X. Dou, D. Yang, Adv. Synth. Catal. 361 (2019) 1808-1814; (b) Y. Zong, Y. Lang, M. Yang, et al., Org. Lett. 21 (2019) 1935-1938; (c) X.Q. Chu, D. Ge, T.P. Loh, Z.L. Shen, Org. Chem. Front. 6 (2019) 835-840; (d) X. Wang, M. Yang, W. Xie, X. Fan, J. Wu, Chem. Commun. 55 (2019) 6010-6013; (e) G.H. Li, D.Q. Dong, Q. Deng, S.Q. Yan, Z.L. Wang, Synthesis 51 (2019) 3313-3319; (f) F.S. He, Y. Wu, X. Li, H. Xia, J. Wu, Org. Chem. Front. 6 (2019) 1873-1878; (g) K. Sun, Z. Shi, Z. Liu, et al., Org. Lett. 20 (2018) 6687-6690; (h) J. Zhang, X. Li, W. Xie, S. Ye, J. Wu, Org. Lett. 21 (2019) 4950-4954; (i) S. Ye, T. Xiang, X. Li, J. Wu, Org. Chem. Front. 6 (2019) 2183-2199. |
| [10] |
P. Bao, L. Wang, H. Yue, et al., J. Org. Chem. 84 (2019) 2976-2983. DOI:10.1021/acs.joc.8b02844 |
| [11] |
W.H. Bao, M. He, J.T. Wang, et al., J. Org. Chem. 84 (2019) 6065-6071. DOI:10.1021/acs.joc.9b00178 |
| [12] |
(a) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. China Chem. 62 (2019) 460-464; (b) Y. You, K. Zhou, B. Guo, et al., ACS Sens. 4 (2019) 774-779; (c) T.Y. Shang, L.H. Lu, Z. Cao, et al., Chem. Commun. 55 (2019) 5408-5419; (d) L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 1237-1240; (e) L. Peng, Z. Hu, Q. Lu, et al., Chin. Chem. Lett. (2019), doi: http://dx.doi.org/10.1016/j.cclet.2019.05.063; (f) Q. Zhu, C. Liu, L. Zhou, et al., Biosens. Bioelectron. 140 (2019) 111356; (g) K.J. Liu, Z.H. Duan, X.L. Zeng, et al., ACS Sustainable Chem. Eng. 7 (2019) 10293-10298; (h) L.Y. Xie, T.G. Fang, J.X.T. Tan, et al., Green Chem. 21 (2019) 3858-3863; (i) L.Y. Xie, L.L. Jiang, J.X. Tan, et al., ACS Sustainable Chem. Eng. 7 (2019) 14153-14160. |
| [13] |
(a) W. Zhao, P. Xie, Z. Bian, et al., J. Org. Chem. 80 (2015) 9167-9175; (b) X. Pang, L. Xiang, X. Yang, R. Yan, Adv. Synth. Catal. 358 (2016) 321-325. |
 2019, Vol. 30
2019, Vol. 30 


