b Environmental Engineering Program, Department of Civil Engineering, Auburn University, Auburn, AL 36849, United States;
c The Beijing Innovation Center for Engineering Science and Advanced Technology(BIC-ESAT), Peking University, Beijing 100871, China;
d Institute of Environmental Science, Taiyuan University of Science and Technology, Taiyuan 030024, China
As a radioactive fission product from 235U (primary) or 239Pu, technetium (99Tc) is released to the environment due to atmospheric nuclear testing and reprocessing of spent fuel, and the increasing inventory of 99Tc is waiting for final disposition [1]. For example, in the accident on the TEPCO's Fukushima Nuclear Power Station in Japan, the discharge amount of 99Tc into the environment is determined as > 0.25 T Bq [2]. Tc concentration in the groundwater can vary from ~50 pCi/L to > 14, 000 pCi/L at the source. If the concentration of 99Tc arises suddenly due to accidental release, widespread contamination of seawaters, rivers, subsurface pore waters, ground waters, and sediments will pose long-time (half-life is 2.14 × 105 years) radiological threat to both plants and animals (lung cancer and related maladies) through uptake and food chain [1]. For public health, the drinking water standard for 99Tc (β-emitter) is 900 pCi/L (or 5.3 × 10-10 mol/L; 33.3 Bq/dm3), suggesting that the amount of 99Tc allowed to be present in subsurface groundwater is considerably smaller. The main form of 99Tc, 99TcO4- (99Tc(Ⅶ)), is quite mobile, so the immobilization of 99Tc represents a key strategy to keep it from spreading in liquid or aquifer phase.
Compared with 99TcO4-, 99Tc(Ⅳ) oxide is insoluble, which is the predominant product of 99Tc(Ⅶ) reduction (Fig. S1 in Supporting information) [3]. Previous works developed many technologies transformation from Tc(Ⅶ) to Tc(Ⅳ) so as to realize the immobilization, such as chemical reduction/precipitation [4-6], biological treatment [7] and photoreduction [8]. Fe-based materials are widely used as reducing agents for 99Tc(Ⅶ), such as zero valent iron (ZVI) [3], FeS [9] and pyrite [10]. However, the ability of Fe(0) and/or Fe(Ⅱ) to reduce or absorb 99Tc(Ⅶ) depends on their physiochemical properties, including surface modification.
During the synthesis of nanoscale ZVI (nZVI) particles, both their spontaneous agglomeration and continued passivation by water molecules once particles formed can largely consume their reduction capacity onto target contaminants. A proper stabilizer is proposed to guarantee nZVI with both larger specific total surface area and much more targeted performance functionally. The food grade starch has been used as stabilizer for bimetallic nanoparticles (~55 m2/g) to degrade Trichloroe-thene (TCE) and polychlorinated biphenyls (PCBs) [11]. In addition, carboxymethyl cellulose (CMC) was also found as an efficient stabilizer to manipulate the size and dispersibility of ZVI particles [12]. The CMC-stabilized nZVI performed much smaller hydrodynamic size (18.6 nm) than bare ZVI (> 3 μm) based on the dynamic light scattering (DLS) method [12], which showed high reactivity on most of heavy metals and organic contaminants compared with unstabilized ZVI due to the protection of formed nanoparticles from agglomeration [13, 14]. Therefore, in this study, soluble starch and CMC are applied as stabilizers to facilitate nZVI preparation, and the removal of 99Tc(Ⅶ) from simulated groundwater by the two stabilized nZVI was compared.
The overall goal of this work was to test the effectiveness of CMC- and starch-stabilized nZVI for reductive sequestration of the highly mobile and soluble 99Tc(Ⅶ) in simulated groundwater. The specific objectives were to: (1) test the removal efficiencies of 99TcO4- by stabilized nZVI with different dosages, (2) study the effects of solution chemistry conditions such as pH, bicarbonate, and natural organic matter (NOM) on Tc immobilization, (3) elucidate the underlying 99Tc(Ⅶ) reduction mechanism by means of material characterizations, and (4) evaluate the long-term stability of reduced products under aerobic and anaerobic circumstances.
All chemicals used in this work were of analytical grade or higher. Details related to the chemicals are provided in Text S1 (Supporting information). CMC- or starch-stabilized ZVI were prepared through our previous reported methods [15-17]. Specifically, 10 mL of 22.3 mmol/L FeCl2 4H2O was added to 25 mL 1 wt% stabilizer (CMC or starch) solution in a flask (250 mL), and the mixture was stirred at 600 rpm for 5 min. Then the flask was immediately sealed and fixed on the shaker and connected with vacuum. Then 10 mL of sodium borohydride solution based on the stoichiometric amounts was injected into the flask for 3 min to yield a dark and evenly distributed particle suspension. The suspension was then shaken for another 2 min followed by 2 min standing, in order to facilitate further growth and refinement of particles. Finally, the stabilized nZVI particle suspension is of 60 mg/L as Fe (also available from 10 mg/L to 100 mg/L) with CMC or starch concentration of 0.2 wt% eventually by mixing with contaminated DI water containing 99TcO4- stock solution.
Batch kinetic experiments were carried out to test the effectiveness of stabilized nZVI nanoparticles for reductive removal of 99Tc(Ⅶ). The initial 99Tc(Ⅶ) concentration was fixed at 12 mg/L with nZVI dosage varying from 10 mg/L to 100 mg/L. Batch experiments were conducted in 30 mL glass vials with Teflon-lined caps, and the vial headspace was near zero to minimize dissolved oxygen (DO) effect. The initial pH was adjusted to 8.0 by 0.1 mmol/L HCl and NaOH. The mixtures were then placed on a shaker (200 rpm) and sampled at pre-determined time intervals and then filtered through a VSWP membrane (mixed cellulose esters, pore size = 25 nm, Merck Millipore Ltd., Germany). Control tests in the presence of CMC, starch, Fe(Ⅱ) or sodium borohydride but without the nanoparticles were also carried out but under other identical conditions and same synthesized procedure. The 99Tc(Ⅶ) concentration in the supernatant was measured by liquid scintillation counter (Beckman LS 6500 Multiple-purpose Scintillation Counter, USA), where each 5 mL Tc sample was mixed with 10 mL of the scintillation cocktail (Ultima-GoldTM, PerkinElmer, Inc., MA, USA). The detection limit of 99Tc was ~5.98 × 10-10 mol/L (15.33 CPM, or 5.92 10-5 mg/L).
Experiments on effects of material dosage, pH, bicarbonate and HA were conducted similarly, and details are presented in the Text S2 (Supporting information). CMC-stabilized nZVI samples before and after 99Tc(Ⅶ) reduction were characterized by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) (Text S3 in Supporting information). A long-term evaluation on reoxidation of reduced Tc was conducted over 120 days, and the method on the stability test on immobilized Tc is provided in the Text S4 (Supporting information).
Given that the removal of 99Tc(Ⅶ) in this study was carried out by stabilized nZVI, control tests of 99Tc(Ⅶ) reduction in the presence of stabilizer (CMC or starch), or sodium borohydride (a strong reducing agent) used in the preparation of the nZVI nanoparticles were also carried out. Fig. S2 (Supporting informa-tion) shows that after 24 h reaction > 99% of 99Tc(Ⅶ) removal was achieved by 60 mg/L CMC-nZVI and starch-nZVI. However, single CMC, starch or sodium borohydride did not show any reduction of 99Tc(Ⅶ) and single Fe(Ⅱ) presented the limited reduction efficiency (18.6%) of 99Tc(Ⅶ), indicating that the observed 99Tc (Ⅶ) reduction was solely attributed to the nZVI nanoparticles. The reason for almost no reduction of 99Tc(Ⅶ) by control NaBH4 solution was due to the experimental procedure, and NaBH4 could quickly react with H2O before Tc(Ⅶ) addition due to its high activity. Detailed discussion was presented in Text S5 (Supporting information).
Fig. 1 shows the reductive removal of 99Tc(Ⅶ) by CMC-stabilized (Fig. 1a) and starch-stabilized nZVI (Fig. 1b) at various dosages (10-100 mg/L as Fe). Rapid and high (> 92%) removal of 99Tc(Ⅶ) was observed within 0.5 h for both CMC- and starch-stabilized nZVI at material dosage > 60 mg/L. The pseudo-first order kinetic model is used to describe the kinetic data (Eq. 1) [18, 19]:
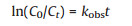
|
(1) |

|
Download:
|
| Fig. 1. Kinetics of 99Tc(Ⅶ) reduction by CMC-stabilized (a) and starch-stabilized nZVI (b). (Initial 99Tc(Ⅶ) = 12 mg/L; nZVI concentration = 10-100 mg/L as Fe; CMC or starch = 0.2 wt%; initial pH 8.0; CTL: control text without material addition). | |
where C0 and Ct are the Tc concentrations (mg/L) at time 0 and t (h) in aqueous phase, respectively; and κobs is the observed first-order rate constant (h-1).
Table S1 (Supporting information) summarizes the best-fitted parameters, and the pseudo-first order model can well describe the kinetic data when material dosage 40 mg/L (R2 > 0.9). The rate constant (κobs) increased with increasing material dosage for both CMC- and starch-stabilized nZVI. Especially, for CMC-nZVI (Fig. 1a), the κobs increased from 0.105 h-1 to 9.179 h-1 (by 87 times) as the material concentration increased from 10 mg/L to 100 mg/L as Fe. The dosage effect was more profound when it increased from 10 mg/L to 20 mg/L, as the κobs sharply raised from 0.105 h-1 to 2.431 h-1 (by 23 times). The mass transfer limited the reactions at higher material dosage. Similarly, for starch-nZVI (Fig. 1b), the κobs was increased from 0.080 h-1 to 8.348 h-1 (by 104 times) as the material concentration increased from 10 mg/L to 100 mg/L as Fe, and quick kinetics were only found when starch-nZVI dosage 60 mg/L. Generally, though both materials can efficiently remove 99Tc(Ⅶ) from the aqueous phase, the reduction rate was higher by CMC-nZVI than by starch-nZVI (Table S1). Therefore, in the following experiments and characterizations, CMC-nZVI was focused. The enhancement of the 99Tc(Ⅶ) reaction rate at higher stabilized nZVI dosage can be attributed to more available reactive sites.
Nano-size ZVI particles are a strong reducing agent and can be much stronger after stabilization [14]. In this way, nZVI nano-particles have relatively large surface area, numerous reaction sites and high chemical reactivity [12]. That explains the consistent quick kinetic and high removal efficiency of 99Tc(Ⅶ) using either CMC- or starch-stabilized nZVI. However, as consequences of high reactivity, a good fraction of reductive capacity of nZVI was consumed by water molecules especially when the particles were freshly prepared [20]. For example, in Fig. 1a, although 10 mg/L CMC-nZVI was adequate to convert initial 12 mg/L 99Tc(Ⅶ) by stoichiometry, more than 99% of 99Tc(Ⅶ) cannot be removed within 24 h. The same phenomenon was observed from Fig. 1b, except that the barrier of rapid kinetics initiated by starch-nZVI was more significant than that by CMC-nZVI, which suggested that the negative charges carried by CMC facilitated not only the repulsion forces between nZVI particles when generating CMC-nZVI but also the attraction from nZVI to 99TcO4-. In addition, CMC-nZVI usually has smaller particle size and higher surface area than starch-nZVI [12]. Meanwhile, starch prevented starch-Fe(Ⅱ) from agglomeration without charging particles [15, 21], ferrous ions also acted as a reducing agent to convert 99Tc(Ⅶ) to 99Tc(Ⅳ), and greater rate and extent of 99Tc(Ⅶ) reduction were promoted by higher amounts of Fe(Ⅱ) at a given pH.
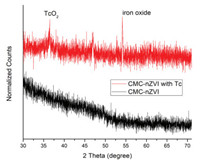
Fig. 2 displays XRD patterns of CMC-nZVI before and after reacting with 99Tc(Ⅶ). There are no significant peaks of assigned to Fe in the CMC-nZVI spectra, which is consistent with previous findings due to the large amount of coated stabilizers (CMC) and less ordered Fe0 structure [14]. After reaction, two distinct crystalline phases appeared, as indicated by the two new characteristic peaks of TcO2 plane (JCPDS No. 00-022-0910) at 36.2 and Fe2O3 (116) plane at 54.2 (JCPDS No. 33-0664), respectively [22-24], suggesting successful reduction of 99Tc (Ⅶ). The weak peak of TcO2 can be due to the low concentration of Tc. The XRD agrees with the Fe0-facilitated redox reaction mechanism illustrated below.
|
Download:
|
| Fig. 2. XRD patterns of CMC-nZVI before and after 99Tc(Ⅶ) reduction. | |
Fig. 3 presents the XPS spectra of CMC-nZVI before and after 99Tc(Ⅶ) reduction, and Table S2 (Supporting information) lists the atomic percentage of the main elements. The main elements of neat CMC-nZVI were Fe (2.4%), O (32%), C (59.1%) and Na (6.5%), where the coated CMC will contribute to the C, O and Na. Furthermore, due to the high reactivity and water erosion, partial oxidation of Fe0 can happen and introduce O to the material [25]. For reduction of 99Tc(Ⅶ) with CMC-nZVI at 12 h, it is clearly show that a Tc 3d peak appeared (Fig. 3a), indicating deposition of Tc reduced products onto nZVI particles. The high resolution of Tc 3d further revealed reduction of 99Tc(Ⅶ) to 99Tc(Ⅳ) (Fig. 3b). Specifically, the peaks at ca. 257.6 eV and 255.7 eV assigned to Tc (Ⅶ) and Tc(Ⅳ), respectively [9, 26], which consists of 80.1% of Tc as Tc(Ⅳ) and 19.9% as remaining Tc(Ⅶ). Therefore, efficient reductive immobilization of 99Tc(Ⅶ) was achieved, and the formed 99Tc(Ⅳ) is widely confirmed as TcO2(s), which can precipitate onto nZVI due to low solubility [3]. 99Tc(Ⅶ) reduction kinetics results, XRD and XPS analysis all indicated that the reduced product, TcO2(s), precipitated onto the Fe nanoparticles, so the Tc removal process is a combination of reduction and precipitation/adsorption.

|
Download:
|
| Fig. 3. XPS spectra of CMC-nZVI before and after reduction of 99Tc(Ⅶ): (a) survey XPS, (b) high resolution of Tc 3d. | |
Fan et al. [27] and Huo et al. [10] observed that the precipitation of the resultant TcO2 under iron reducing conditions. Based on these observations and the above experimental results, the reductive immobilization of 99Tc(Ⅶ) by stabilized nZVI is contributed by: (1) direct sorption of 99TcO4- on the nanoparticle surface, (2) Fe0 -mediated heterogeneous reduction, and (3) chemical co-precipitation of the resultant TcO2. Fig. 4 illustrates the schematic diagram of 99Tc(Ⅶ) reduction on the CMC-stabilized nZVI. The following Eq. 2 depicts the redox reaction:

|
(2) |

|
Download:
|
| Fig. 4. Schematic diagram on 99Tc(Ⅶ) reductive immobilization and remobilization on the CMC-stabilized nZVI. | |
Meanwhile, Ponder et al. claimed that the nZVI nanoparticles (Fe0) can also react with water to form Fe(Ⅱ) ions as (Eqs. 3-5) [20, 28]:
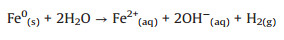
|
(3) |
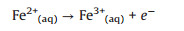
|
(4) |

|
(5) |
The following homogeneous redox reaction between Fe(Ⅱ) and 99Tc(Ⅶ) can also occur (Eq. 6) [21]. However, in the heterogeneous Fe(Ⅱ) reaction system, i.e., adsorbed ferrous ions on the surface of nZVI, 99Tc(Ⅶ) will be reduced to 99TcO2 as Eq. 7 [29].
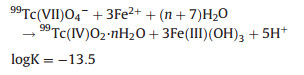
|
(6) |
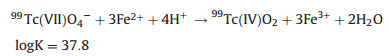
|
(7) |
where K is the equilibrium constant under standard conditions.
Besides direct reduction of 99Tc(Ⅶ) by Fe0, Eqs. 5 and 7 also reveal that the indirect formation of 99Tc(Ⅳ)O2(s), and the reaction is strongly pH-dependent and favors at low pH.
The rate and extent of 99Tc(Ⅳ) dissolution was highly related to the organic ligands of chemical and structural properties [30], hence also causing significant implications for the 99Tc(Ⅶ) reduction. Furthermore, as a common buffer and a popular composition in natural subsurface water, natural organic matter (e.g., humic acid) and high concentration of bicarbonate was usually found in natural water matrix [31]. Based on the pseudo-first order model fitting, Fig. S3 (Supporting information) displays the reduction rate constants (κobs) in the absence and presence of bicarbonate and HA at various pH. It was evident that bicarbonate showed minor effect while HA inhibited the reduction reaction of 99Tc(Ⅶ). 50 mg/L HA retarded the reduction as the κobs value decreased from ~0.2 h-1 to ~0.1 h-1 (by 2 times) in all the pH range from 5.0 to 8.0. However, additional 10 mmol/L HCO3- - only slightly decreased the reaction rate at pH 5.0 and 6.0.
As shown in Eqs. 5 and 7, H+ plays an important role in the reduction of 99Tc(Ⅶ), and the redox reaction is highly pH-dependent. In addition, Tc(Ⅳ)O2(s) is more favorable to form at lower pH (Fig. S1). However, reductive removal of 99Tc(Ⅶ) will be affected by pH in some contrasting ways. pH can strongly affect the surface charge of CMC-nZVI and then involve in the reaction between nanoparticles and 99Tc(Ⅶ). He et al. [32] reported that CMC-nZVI was negatively charged at pH 5.0-8.0, which is consistent with pKa value of 4.3 of CMC, suggesting the CMC-stabilized nZVI showed electrostatic repulsion effect on 99TcO4-. The κobs value slightly decreased from 0.193 h-1 to 0.180 h-1 when pH increased from 5.0 to 6.0, due to more negative chargers at pH 6.0 and was not favorable for 99TcO4- adsorption. In addition, Eqs. (5) and (7) indicated that higher concentration of H+ also facilitate formation of Tc(Ⅳ)O2(s). However, the κobs value then increased to 0.201 h-1 at pH 8.0, and it is mainly due to higher corrosion rate of Fe0 at lower pH. He and Zhao [12] reported that the corrosion rate of CMC-nZVI increased by ~31.4 times from pH 8.2 to 6.0. Therefore, higher corrosion rate at lower pH results in the partial loss of reactive sites of nZVI, leading to lower 99Tc(Ⅶ) removal efficiency.
The inhibition effect of HA on CMC-nZVI reactivity for reduction of 99Tc(Ⅶ) contains various mechanisms [33]. First, the negative charged HA will compete surface reactive sites of CMC-nZVI with 99TcO4-, resulting in a protective and nonconductive layer, which cut off the electron transfer and thus inhibited 99Tc(Ⅶ) reduction [34]. Secondly, HA can not only be adsorbed on the surface of particles but also react with Tc(Ⅵ)O2·H2O colloids. Boggs et al. [35] reported that the presence of HA may re-dissolve the reduced Tc (Ⅳ) species due to the formation of [Tc(OH)2]-HA complexes, resulting in remobilization of 99 Tc(Ⅶ). Thirdly, HA can also easily bond with 99TcO4- in solution and form stable HA-Tc complex [35], which is hard to reduction. Similarly, complexation between HA and Fe 2+ or Fe3+ will inhibit the electron transfer and then affect the reduction reaction [36].
In the presence of bicarbonate, the corrosion of Fe0 by HCO3- will occur [37]. The added bicarbonate can be sorbed onto CMC-nZVI surface and react as oxidant following the reaction (Eq. 8) [38]:
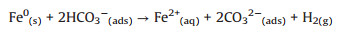
|
(8) |
The precipitated carbonate can also coat on the iron surface thereby inhibit the further reaction [38, 39]. Severe corrosion of nZVI generally occurs at low pH [38, 39], so the reduction of 99Tc(Ⅶ) was only slightly inhibited in this study when pH varied from 6.0 to 8.0.
With rapid rate and high remove efficiency in the short term, the CMC-stabilized nZVI is considered as a promising reductive agent for remediation of 99Tc contaminated groundwater. However, the factor of oxygen cannot be ignored or completely excluded from the 99Tc(Ⅶ) reduction in natural environment or artificial perturbations. To simulate this process, a set of batch experiments regarding long-term monitor on reduction stability and reoxidation fraction were carried out.
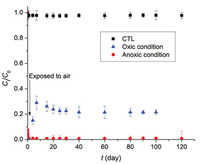
Fig. 5 shows the redissolution or remobilization of 99Tc(Ⅶ) after complete reduction of 99Tc(Ⅶ) by CMC-nZVI for 120 d under both anoxic and oxic conditions. Without air involved, which simulate typical subsurface redox environment, no Tc remobilization was detected during the 120 d of aging. This observation suggested that the immobilized 99Tc(Ⅳ) remained highly stable over prolonged period of time under anoxic condition. However, with air involved and exposed to the atmospheric condition, the immobilized Tc was partially remobilized, with a peak of soluble Tc concentration accounting to 29.1% of the total Tc in the system. The peak of dissolved Tc concentration occurred at day 7, and then gradually decreased to a steady concentration (21.5% of total Tc) after 20 d, which can be attributed to the slow adsorption by iron oxyhydroxides resulting from oxidation of Fe0 [20] and radiolytic auto-reduction of 99TcO4- to 99TcO2. The largely irreversible reduction of 99Tc(Ⅶ) by CMC-nZVI is desirable in practical applications, and it can be attributed to: (1) The structural barrier effect of the nanoparticles on Tc that is situated in the pores [40], and (2) reoxidation of the immobilized Tc(Ⅳ)O2(s) in the solid phase needs much more activation energy than the reductive sequestration of Tc(Ⅶ). In addition, the protective effect will be addressed due to the formation of oxidized layer [41], such as iron (hydr)oxides.
|
Download:
|
| Fig. 5. Long term evaluation on stability of 99Tc(Ⅶ) under anoxic and oxic conditions. (Initial 99Tc(Ⅶ) = 12 mg/L; nZVI concentration = 60 mg/L as Fe; CMC = 0.2 wt%; initial pH 8.0; CTL: control text with CMC and 99Tc(Ⅶ) but without material addition). | |
This study investigated the feasibility of reductive immobilization 99TcO4- using stabilized nZVI nanoparticles in water. CMC-stabilized nZVI performed much faster kinetics of 99Tc(Ⅶ) reduction than starch-stabilized particles, especially when the nZVI dosage was < 60 mg/L, mainly due to the extra charges carried by CMC molecules minimized the diffusion barrier as to accelerate effective contacts between CMC-nZVI and 99TcO4-. XRD and XPS analyses demonstrated formation of 99TcO2(s) after reduction. The reductive reaction of 99Tc(Ⅶ) was a pH-indepen-dent process, the co-existing HA inhibited the reaction while bicarbonate showed minor effect. Extremely stable immobilized Tc was observed under anoxic condition even after 120 d, and only 21% reoxidation of aged 99Tc(Ⅳ) was found after ingress of atmospheric O2, which further supported that the CMC-nZVI was an innovative alternative for radionuclides groundwater remediation.
AcknowledgmentsThis work was partially supported by the National Natural Science Foundation of China (No. 41230638), and a grant from the USDA AAES 2015 Hatch and Multistate funding program.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.06.004.
| [1] |
J.P. Icenhower, N.P. Qafoku, J.M. Zachara, W.J. Martin, Am. J. Sci. 310 (2011) 721-752. DOI:10.2475/08.2010.02 |
| [2] |
K.L. Shi, X.L. Hou, P. Roos, W.S. Wu, Anal. Chim. Acta 709 (2012) 1-20. DOI:10.1016/j.aca.2011.10.020 |
| [3] |
J.G. Darab, A.B. Amonette, D.S.D. Burke, et al., Chem. Mater. 19 (2007) 5703-5713. DOI:10.1021/cm0607379 |
| [4] |
Y. Liu, J. Terry, S.S. Jurisson, Radiochim. Acta 96 (2008) 823-833. |
| [5] |
T. Peretyazhko, J.M. Zachara, S.M. Heald, et al., Geochim. Cosmochim. Acta 72 (2008) 1521-1539. DOI:10.1016/j.gca.2008.01.004 |
| [6] |
Y. Wang, C. Wang, Chin. Chem. Lett. 29 (2018) 345-352. DOI:10.1016/j.cclet.2018.01.001 |
| [7] |
L. Newsome, A. Cleary, K. Morris, J.R. Lloyd, Environ. Sci. Technol. 51 (2017) 1595-1604. DOI:10.1021/acs.est.6b04876 |
| [8] |
Burton-Pye B.P., R. Ivana, M.G. Donna, et al., J. Am. Chem. Soc. 46 (2011) 18802-18815. |
| [9] |
H. Ji, Y. Zhu, W. Liu, et al., Colloids and Surfaces A 561 (2019) 373-380. DOI:10.1016/j.colsurfa.2018.10.048 |
| [10] |
L. Huo, W. Xie, T. Qian, X. Guan, D. Zhao, Chemosphere 174 (2017) 456-465. DOI:10.1016/j.chemosphere.2017.02.018 |
| [11] |
F. He, D.Y. Zhao, Environ. Sci. Technol. 39 (2005) 3314-3320. DOI:10.1021/es048743y |
| [12] |
F. He, D.Y. Zhao, Environ. Sci. Technol. 41 (2007) 6216-6221. DOI:10.1021/es0705543 |
| [13] |
F. He, D. Zhao, J. Liu, C.B. Roberts, Ind. Engin. Chem. Res. 46 (2007) 29-34. DOI:10.1021/ie0610896 |
| [14] |
X. Zhao, W. Liu, Z. Cai, et al., Water Res. 100 (2016) 245-266. DOI:10.1016/j.watres.2016.05.019 |
| [15] |
Q. Liang, D. Zhao, T. Qian, K. Freeland, Y. Feng, Ind. Engin. Chem. Res. 51 (2012) 2407-2418. DOI:10.1021/ie201801d |
| [16] |
Z. Xiong, D. Zhao, G. Pan, Water Res. 41 (2007) 3497-3505. DOI:10.1016/j.watres.2007.05.049 |
| [17] |
H.F. Liu, T.W. Qian, D.Y. Zhao, Chin. Sci. Bull. 58 (2013) 275-281. DOI:10.1007/s11434-012-5425-3 |
| [18] |
M.J. Alowitz, M.M. Scherer, Environ. Sci. Technol. 36 (2002) 299-306. DOI:10.1021/es011000h |
| [19] |
Q. Chen, L. Chen, J. Qi, et al., Chin. Chem. Lett. 30 (2019) 1214-1218. DOI:10.1016/j.cclet.2019.03.002 |
| [20] |
S.M. Ponder, J.G. Darab, J. Bucher, et al., Chem. Mater. 13 (2001) 479-486. DOI:10.1021/cm000288r |
| [21] |
J.M. Zachara, S.M. Heald, B.H. Jeon, et al., Geochim. Cosmochim. Acta 71 (2007) 2137-2157. DOI:10.1016/j.gca.2006.10.025 |
| [22] |
Z. Liu, B.L. Lv, Y. Xu, D. Wu, J. Mater. Chem. A 1 (2013) 3040-3046. DOI:10.1039/c2ta00987k |
| [23] |
G.Q. Zhang, X. Zhang, T. Lin, T. Gong, M. Qi, Chin. Chem. Lett. 23 (2012) 145-148. DOI:10.1016/j.cclet.2011.10.015 |
| [24] |
Z. Wang, T.F. Zhang, Z. Ge, Y.J. Luo, Chin. Chem. Lett. 26 (2015) 1535-1537. DOI:10.1016/j.cclet.2015.07.017 |
| [25] |
J. Duan, H. Ji, W. Liu, et al., Chem. Eng. J. 359 (2019) 1617-1628. DOI:10.1016/j.cej.2018.11.008 |
| [26] |
D.W. Wester, D.H. White, F.W. Miller, et al., Inorg. Chim. Acta 131 (1987) 163-169. DOI:10.1016/S0020-1693(00)96019-5 |
| [27] |
D. Fan, R.P. Anitori, B.M. Tebo, et al., Environ. Sci. Technol. 47 (2013) 5302-5310. DOI:10.1021/es304829z |
| [28] |
H. Zhang, Q. Ji, L. Lai, G. Yao, B. Lai, Chin. Chem. Lett. 30 (2019) 1129-1132. DOI:10.1016/j.cclet.2019.01.025 |
| [29] |
J. Yang, R.K. Kukkadapu, H. Dong, et al., Chem. Geol. 291 (2012) 206-216. DOI:10.1016/j.chemgeo.2011.10.013 |
| [30] |
B. Gu, W. Dong, L. Liang, N.A. Wall, Environ. Sci. Technol. 45 (2011) 4771-4777. DOI:10.1021/es200110y |
| [31] |
F.W. Trainer, R.C. Heath, J. Hydrol. 31 (1976) 37-55. DOI:10.1016/0022-1694(76)90019-6 |
| [32] |
C. He, Y. Hu, L. Yin, C. Tang, C. Yin, Biomater. 31 (2010) 3657-3666. DOI:10.1016/j.biomaterials.2010.01.065 |
| [33] |
M. Zhang, F. He, D. Zhao, X. Hao, Water Res. 45 (2011) 2401-2414. DOI:10.1016/j.watres.2011.01.028 |
| [34] |
A. Maes, K. Geraedts, C. Bruggeman, et al., Environ. Sci. Technol. 38 (2004) 2044-2051. DOI:10.1021/es034720s |
| [35] |
M.A. Boggs, Technetium(Ⅳ) complexation with organic ligands, Dissertation, Washington State University, 2012.
|
| [36] |
S. Kumar, N. Rawat, A.S. Kar, B.S. Tomar, V.K. Manchanda, J. Hazard. Mater. 192 (2011) 1040-1045. DOI:10.1016/j.jhazmat.2011.06.007 |
| [37] |
J.T. Nurmi, P.G. Tratnyek, Corros. Sci. 50 (2008) 144-154. DOI:10.1016/j.corsci.2007.06.016 |
| [38] |
A. Agrawal, W.J. Ferguson, B.O. Gardner, et al., Environ. Sci. Technol. 36 (2002) 4326-4333. DOI:10.1021/es025562s |
| [39] |
J. Klausen, P.J. Vikesland, T. Kohn, et al., Environ. Sci. Technol. 37 (2003) 1208-1218. DOI:10.1021/es025965s |
| [40] |
T. Peretyazhko, J.M. Zachara, S.M. Heald, et al., Geochim. Cosmochim. Acta 72 (2008) 1521-1539. DOI:10.1016/j.gca.2008.01.004 |
| [41] |
D. Fan, R.P. Anitori, B.M. Tebo, et al., Environ. Sci. Technol. 48 (2014) 7409-7417. DOI:10.1021/es501607s |
 2019, Vol. 30
2019, Vol. 30 

