b School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China;
c Department of Pharmacy, Yantai Univeristy, Yantai 264005, China
Oxidative stress, which is mainly caused by the imbalance between cellular antioxidant response systems and reactive oxygen species (ROS) production, results in DNA damage, lipid peroxidation and even cell death [1]. It has been revealed that ROS are a chemically heterogenic group of highly reactive molecules and they are naturally produced by metabolic redox reactions of biological systems [2, 3]. Accumulating evidence suggested that ROS are responsible for human's aging and various pathologies such as inflammation, diabetes, rheumatoid arthritis, Alzhimer's disease and carcinogenesis [4-7]. The study on the significance of ROS in the pathogenesis of various diseases and the research for the discovery of novel, small molecules as antioxidant agents have attracted uninterrupted attention.
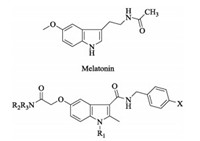
As known, the basic heterocyclic structures play a significant role in the drug design. Among them, indole derivatives have drawn great attention due to their excellent biological activities. It was reported that melatonin (Fig. 1) and its derivatives showed antioxidant property in both in vitro and in vivo models [8-10]. Melatonin is an endogenous chemical mediator excreted by pineal gland according to a circadian rhythm. And it has low toxicity and limitation in pharmacokinetic issues just like short half-life and bioavailability [11-14]. Therefore, melatonin was chosen as a lead compound in the present work and some structural modifications were devoted to preliminarily evaluate the antioxidant activities of melatonin derivatives.
|
Download:
|
| Fig. 1. Chemical structures of N-acetyl-5-methoxytryptamine (Melatonin) and the target indole-3-carboxamide derivatives. | |
In this study, twelve novel indole-3-carboxamide derivatives (Fig. 1) as melatonin analogs were obtained, and their in vitro antioxidant activities were evaluated via H2O2 radical scavenging tests.
The synthetic routes of the target compounds are depicted in Scheme 1. Intermediate 2a or 2b was obtained by reactions of the commercially available ethyl acetoacetate (1) with methylamine or ethylamine at ambient temperature, which was converted to indole-3-carboxylate 3a or 3b via Nenitzescu reaction with benzoquinone. Hydrolysis of 3a or 3b using sodium hydroxide in ethanol and water gave indole-3-carboxylic acid 4a or 4b. Treatment of 4a or 4b with benzylamine or 4-fluorobenzylamine in the presence of EDCI and HOBt provided key intermediates 5a-5d. Reaction of 5a-5d with corresponding 2-chloroacetamide derivatives by using anhydrous K2CO3 and KI gave indole-3-carboxamide derivatives 6a-6l, respectively. The details are given in the Supporting information.

|
Download:
|
| Scheme 1. Synthetic route of the target indole-3-carboxamide derivatives. Reagents and conditions: (a) R1NH2, r.t., 3 h; (b) benzoquinone, acetone, 30 ℃, 2 h; (c) NaOH, H2O, ethanol, reflux, 12 h; (d) EDCI, HOBt, triethylamine, DMF, CH2Cl2, r.t., 24 h; (e) ClCH2CONR2R3, K2CO3, KI, DMF, 80 ℃, 16 h. | |
Using ascorbic acid as a control, the target compounds 6a-6l were evaluated for the antioxidant activities in vitro against human neuroblastoma SH-SY5Y cell line by H2O2 radical scavenging assay [15-17], and the procedures are given in the Supporting information. The results expressed as cell viability were summarized in Table 1.
|
|
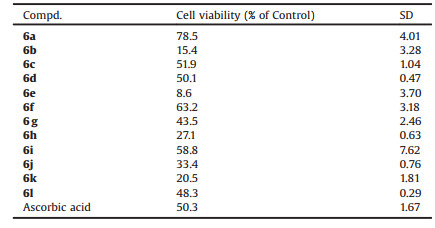
Table 1 In vitro cell viability of the target compounds. |
The SARs analysis revealed as follows: (1) Compared to the target compound 6g, the target compound 6a exhibited better antioxidant activity, which implies methyl in 1-position of indole structure is superior to ethyl; (2) The target compound 6a exhibited better activity than the target compound 6d. These results show that the introduction of 4-fluorobenzylamino group in 3-position resulted in a decrease or loss of the activity; (3) By comparison, compounds 6a-6c showed variant activity. In general, the introductions of 2-oxo-2-(piperidin-1-yl)ethoxy and [2-(2-methoxyphenoxy)ethyl]amino group exhibit strong antioxidant activity, However, the introduction of 2-(morpholin-4-yl)-2-oxoethoxy in to the 5-position results in a decrease or loss of the activity; (4) The target compounds 6a (Fig. 2a), 6f (Fig. 2b) and 6i (Fig. 2c) indicated better activity than the positive control, and 6a exhibited the best antioxidant activity.

|
Download:
|
| Fig. 2. Effect of 6a (a), 6f (b) and 6i (c) on ROS production induced by H2O2 in SH-SY5Y cells, the statistical significance was calculated (*: P < 0.05; **: P < 0.01; ***: P < 0.001). | |
In conclusion, a novel series of indole-3-carboxamide derivatives were designed and synthesized. All of the target compounds were evaluated for the antioxidant activity in vitro against human neuroblastoma SH-SY5Y cell line via H2O2 radical scavenging assay. The target compounds 6a, 6f and 6i demonstrated excellent strong antioxidant activity. What is more, a new antioxidant scaffold and a preliminary discussion on their SARs provide promising opportunities to guide further research on indole-3-carboxamide derivatives as novel antioxidant agents.
AcknowledgmentsThis work was supported by the Nationa Natural Science Foundation of China (NSFC) (No. 21342006), the Program for Innovative Research Team of the Ministry of Education of China (No. IRT_14R36).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.044.
| [1] |
D. Lee, K.Y. Ry, Biochem. Biophys. Res. Commun. 485 (2017) 234-240. DOI:10.1016/j.bbrc.2017.02.105 |
| [2] |
L. Zuo, T. Zhou, B.K. Pannell, A.C. Ziegler, T.M. Best, Acta Physiol. Oxf. (Oxf) 214 (2015) 329-348. DOI:10.1111/apha.12515 |
| [3] |
J.I. Pagel, E. Deindl, Int. J. Mol. Sci. 13 (2012) 13104-13117. DOI:10.3390/ijms131013104 |
| [4] |
C. Hoelzl, J. Bichler, F. Ferk, et al., J. Physiol. Pharmacol. 56 (2005) 49-64. |
| [5] |
H.L. Wang, Z.Y. Yang, B.D. Wang, Transit. Metal Chem. 31 (2006) 470-474. DOI:10.1007/s11243-006-0015-3 |
| [6] |
Y. Zhang, B. Zou, Z. Chen, et al., Bioorg. Med. Chem. Lett. 21 (2011) 6811-6815. DOI:10.1016/j.bmcl.2011.09.029 |
| [7] |
F. Rostami-Charati, Chin. Chem. Lett. 25 (2014) 169-171. DOI:10.1016/j.cclet.2013.09.016 |
| [8] |
M. Mor, C. Silva, F. Vacondio, et al., J. Pineal Res. 36 (2004) 95-102. DOI:10.1046/j.1600-079X.2003.00102.x |
| [9] |
M. Karbownik, E. Gitto, A. Lewiñski, R.J. Reiter, J. Cell. Biochem. 81 (2001) 693-699. DOI:10.1002/jcb.1100 |
| [10] |
C. Phiphatwatcharaded, P. Puthongking, P. Chaiyarit, et al., Arch. Oral Biol. 9 (2017) 55-61. |
| [11] |
D. Bonnefont-Rousselot, F. Collin, Toxicology 278 (2010) 55-67. DOI:10.1016/j.tox.2010.04.008 |
| [12] |
Y. Guney, A. Hicsonmez, C. Uluoglu, et al., Braz. J. Med. Biol. Res. 40 (2007) 1305-1314. DOI:10.1590/S0100-879X2006005000156 |
| [13] |
S. Cuesta, R. Kireev, C. García, et al., Mech. Ageing Dev. 132 (2011) 573-582. DOI:10.1016/j.mad.2011.10.005 |
| [14] |
F. Radogna, M. Diederich, L. Ghibelli, Biochem. Pharmacol. 80 (2010) 1844-1852. DOI:10.1016/j.bcp.2010.07.041 |
| [15] |
A. Maheshwari, M.M. Misro, A. Aggarwal, R.K. Sharma, D. Nandan, FEBS J. 276 (2009) 870-881. DOI:10.1111/j.1742-4658.2008.06831.x |
| [16] |
K.X. Huang, Y.B. Feng, W. Yao, et al., Chin. Chem. Lett. 20 (2009) 1187-1190. DOI:10.1016/j.cclet.2009.04.026 |
| [17] |
J.D. Xu, L.W. Zhang, Y.F. Liu, Chin. Chem. Lett. 24 (2013) 223-226. DOI:10.1016/j.cclet.2013.01.016 |
 2019, Vol. 30
2019, Vol. 30