Cancer, as a disease of extremely high mortality, severely affects health of human [1]. The mainstream treatment for cancer includes surgery, chemotherapy and radiotherapy [2], however, these methods still suffer from their severe toxic side effects to normal tissues and limited specificities to cancer cells [3]. Nowadays, photothermal therapy (PTT) emerges as a novel approach for cancer therapy, which is non-invasive and converts laser energy into thermal energy in the presence of photothermal agents (PTAs) [4-7]. Recently, many PTAs, including Au nanomaterials [8-10], carbon nanomaterials [11-13], copper sulfide nanoparticles [14] and near-infrared (NIR) dye-based nanocarriers [15, 16] have been developed for PTT. Although these PTAs have exhibited some advantages for cancer therapy, their applications are limited by complex synthetic protocols, low efficiency and potential insecurity. Therefore, PTAs which are simple, safe, and suitable for clinic application are needed to expand the practical application of photothermal therapy.
Magnetite (Fe3O4) is a kind of PTAs with excellent biocompatibility and efficiency [17, 18], ferumoxytol has been approved by the Food and Drug Administration (FDA) for treating iron deficiency, and other iron oxide nanoparticles have been used as contrast agents for magnetic resonance imaging and drug carriers [19]. Moreover, magnetite nanoparticles are fascinating for their outstanding magnetic property, which is a basic property for ferrite nanoparticles. Rapid magnetic responsiveness enables ferrite nanoparticles to be used in many biomedical fields such as proteomics and peptidomics analysis, including protein digestion, enrichment of low-abundance peptides/proteins [20]. Recently, Peng et al. reported that magnetite particles stabilized by organic polymer such as polyethylene glycol (PEG) could obtain more stable photothermal effect than traditional Fe3O4 stabilized by small molecules like citrate [21], because magnetite particles stabilized by citrate would gradually oxidize to maghemite, resulting in the reduction of photothermal effect. These liganddirected photothermal properties inspired us to explore how the photothermal effect of magnetite will be after introducing other metal element. Considering magnetic ferrites have similar synthesis method and metal-doped property [22, 23], we synthesized three kinds of ferrite MFe2O4 (M = Fe, Mn or Zn) nanoparticles with uniform size and systematically investigated their photothermal performance.
Owe to the high NIR absorbance of MFe2O4 nanoparticles, three kinds of ferrite nanoparticles showed good photothermal effects when just prepared. However, after preservation for 70 days, the photothermal effect of Fe3O4 and MnFe2O4 nanoparticles observably reduced, meanwhile, the ZnFe2O4 nanoparticles still maintained a relatively stable and excellent photothermal effect. XPS analysis about ratio of Fe2+ and Fe3+ was then conducted to explain the phenomenon [24, 25]. Subsequently, the photothermal effect was evaluated in vitro, the result showed that ZnFe2O4 was a kind of effective PTA and showed no obviously toxicity, exhibiting immense potential in cancer therapy. We expect that our research on photothermal effect of ferrite nanoparticles could inspire further study about photothermal agents for PTT. Furthermore, as PTAs, MFe2O4 nanoparticles can be used with chemotherapy, this chemo/photothermal synergistic therapy always enhances the treatment effect.
The ferrite nanoparticles were synthesized according to the published solvothermal method with a slightly modification [23]. The detailed morphological and structural features of MFe2O4 were examined by transmission electron microscope (TEM), demonstrating a rough surface with the particle size of ~180 nm (Fig. 1a). Meanwhile, field emission scanning electron microscope (FESEM) images also verified the uniform size of MFe2O4 nanoparticles (Fig. 1a). The hydrodynamic diameter (Dh) of MFe2O4 particles was ~200 nm (Fig. 1b and Table S1 in Supporting information) with a narrow polydispersity index (PDI) (Table S1). The uniform size of MFe2O4 implied that the addition of manganese or zinc element did not affect the size and morphology of the nanoparticles, as well as the zeta potential (Fig. S1 in Supporting information). The X-ray photoelectron spectroscopy (XPS) analysis (Fig. 1c) was performed to determine the element composition of the MFe2O4. Results from XPS spectra of the MFe2O4 nanoparticles showed that the three different kinds of MFe2O4 contains C, O and Fe element, moreover, the Mn and Zn element appeared in MnFe2O4 and ZnFe2O4 nanoparticles, these results confirmed the purity of the MFe2O4 products. Inductively coupled plasma-atomic emission spectroscopy (ICP-AES) reveals that the Mn/Fe ratio in MnFe2O4 and Zn/Fe ratio in ZnFe2O4 are 1:8 and 1:9 respectively, which were lower than their stoichiometric ratios. The result was ascribed to the different nucleation and growth kinetics of metal precursors, implying that the M/Fe ratio reduced with the increasing reaction time [26]. The crystalline structures of MFe2O4 nanoparticles were characterized by wide-angle X-ray diffraction (XRD), as shown in the Fig. 1d, patterns could be easily indexed to Fe3O4 (JCPDS No. 75-1609), MnFe2O4 (JCPDS No. 74-2403) and ZnFe2O4 (JCPDS No. 22-1012). In addition, the magnetic hysteresis curves showed no evident remanence and coercivity, suggesting superparamagnetic property of these MFe2O4 nanoparticles (Fig. 1e) [27]. Moreover, the saturation magnetization (Ms) values of MnFe2O4 and ZnFe2O4 were higher than that of Fe3O4, implying greater usage potential such as tumor specific homing under external magnetic field.

|
Download:
|
| Fig. 1. (a) TEM images of (ⅰ) Fe3O4, (ⅱ) MnFe2O4, (ⅲ) ZnFe2O4 nanoparticles, the insets are corresponding FESEM images, the scale bar is 100 nm. (b) Dynamic light scattering curves of MFe2O4 particles in aqueous solution. (c) XPS spectrum of MFe2O4 nanoparticles. (d) The XRD patterns of Fe3O4, MnFe2O4 and ZnFe2O4 nanoparticles. (e) Magnetic hysteresis curves of MFe2O4 nanoparticles. | |
To study the photothermal effect of these three kinds of MFe2O4 nanoparticles, fresh Fe3O4, MnFe2O4 and ZnFe2O4 nanoparticles were separately treated by NIR laser irradiation (λ = 808 nm, 3 W/cm2) at a concentration of 100 μg/mL. As seen from Fig. 2a, the temperatures of fresh Fe3O4, MnFe2O4 and ZnFe2O4 nanoparticles were increased by 29.1, 26.5 and 36.8 ℃, respectively, indicating that these three MFe2O4 nanoparticles are qualified PTAs. Meanwhile, the photothermal effect of fresh ZnFe2O4 nanoparticles was better than that of fresh Fe3O4 and MnFe2O4 nanoparticles. UV–vis spectrum was utilized to investigate the NIR absorption of MFe2O4 dispersion. As shown in the Fig. 2b, we can find that the NIR absorption of ZnFe2O4 nanoparticles was slightly higher than that of Fe3O4 and MnFe2O4 particles. Moreover, according to the previous method [20], the photothermal conversion efficiency (η) of Fe3O4, MnFe2O4 and ZnFe2O4 particles was calculated to be 25.9%, 36.1% and 37.7%, respectively (Table S2 in Supporting information), lower than that of some new type of PTAs such as antimonene quantum dots [28], ultrathin Bi2Se3 nanosheets [29], graphene oxide/black phosphorus nanoflake [30], and ultrathin boron nanosheets [31]. These results demonstrated that the addition of manganese or zinc element can effectively improve the photothermal conversion effect of magnetic nanoparticles. Hence, the higher NIR absorbance and photothermal conversion efficiency ensured the higher photothermal effect of ZnFe2O4 nanoparticles.

|
Download:
|
| Fig. 2. (a) The photothermal effects of Fe3O4, MnFe2O4 and ZnFe2O4 aqueous dispersions at the concentration of 100 μg/mL measured by laser irradiation (λ = 808 nm, 3 W/cm2). H2O is used as a negative control. (b) The change in Vis-NIR absorbance of Fe3O4, MnFe2O4 and ZnFe2O4 nanoparticles at the concentration of 100 μg/mL. (c) The photothermal effects of ZnFe2O4 dispersion at different concentrations measured by laser irradiation (λ = 808 nm, 3 W/cm2) for 500 s. (d) The change in photothermal effect of Fe3O4, MnFe2O4 and ZnFe2O4 nanoparticles at the concentration of 100 μg/mL measured by laser irradiation (λ = 808 nm, 3 W/cm2) for 500 s. | |
Subsequently, the detailed photothermal effects of the MFe2O4 nanoparticles were investigated by being treated with NIR laser irradiation (λ = 808 nm, 3 W/cm2). As shown in Fig. 2c, the photothermal effect of the ZnFe2O4 particles increased with the increasement of concentration, and the temperature rises for different concentration were determined to be 17.7, 23.0, 36.8 and 46.3 ℃, respectively. Correspondingly, the NIR absorbance of ZnFe2O4 particles at 808 nm was detected as 0.058, 0.175, 0.355 and 0.724 (Fig. S2 in Supporting information). Likewise, the similar trend was found for Fe3O4 and MnFe2O4 nanoparticles. NIR absorption at 808 nm (Fig. S2) and photothermal effect (Fig. S3 in Supporting information) improved with the increasement of concentration. Hence, the MFe2O4 nanoparticles have a good photothermal effect, especially for the ZnFe2O4 nanoparticles, which exhibited a superior photothermal effect.
During the experiment, we found that the colour of MFe2O4 nanoparticles changed with increasing storage time (Fig. S4 in Supporting information). After 70 days, the colour of Fe3O4 and MnFe2O4 particles turned from black to brown, while the colour of ZnFe2O4 particles still kept black. Inspired by the amazing change of colour, we tested the photothermal effect of MFe2O4 nanoparticles. The results were shown in Fig. 2d, the temperature change of ZnFe2O4 particles showed slight decrease from 36.8 ℃ to 31.6 ℃, nevertheless the temperature changes of Fe3O4 nanoparticles observably decreased from 29.1 ℃ to 7.3 ℃ while that of MnFe2O4 nanoparticles decreased from 26.5 ℃ to 8.7 ℃, demonstrating that ZnFe2O4 nanoparticles possessed more stable photothermal property than Fe3O4 and MnFe2O4 nanoparticles. Discussed in our previous work [20], the photothermal effect of MFe2O4 nanoparticles is revealed to increase with the increasement of size, which is attributed to redshift of Vis-NIR spectrum. Sizes of these three kinds of nanoparticles in TEM and FESEM images were still about ~180 nm (Fig. S5 in Supporting information) and the hydrodynamic diameter was still ~200 nm after preservation for 70 days, even for 210 days (Fig. S1a and Table S1). Meanwhile, zeta potential of MFe2O4 nanoparticles hardly changed (Fig. S1b) and PDI kept in a narrow range (Table S1).
Therefore, the size of MFe2O4 nanoparticles hardly changed within 70 days, demonstrating that size did not contribute on change of photothermal effect. Moreover, the photothermal conversion efficiency was calculated to be 6.3%, 6.7% and 25.6% for Fe3O4, MnFe2O4 and ZnFe2O4 nanoparticles, respectively (Table S2). Meanwhile, we investigated the NIR absorption of MFe2O4 aqueous dispersion with a concentration of 100 μg/mL. As shown in Fig. 2b, the NIR absorption of ZnFe2O4 dispersion slightly declined from 0.355 to 0.296, whereas the Fe3O4 and MnFe2O4 dispersion significantly decreased from 0.333 to 0.211 and from 0.297 to 0.176 after preservation for 70 days. As our group previously reported [20], Fe3O4 nanoparticles had a strong absorption at 808 nm, however, Fe2O3 nanoparticles had negligible absorption at 808 nm. So we considered that the different performance among these three MFe2O4 nanoparticles resulted from that the Fe2+ oxidized to Fe3+.
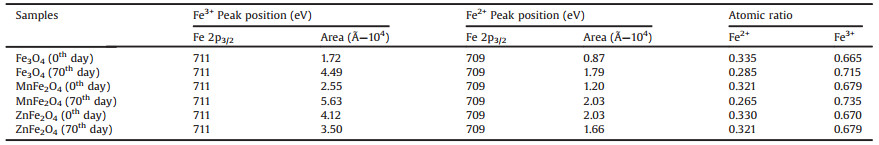
To further study the detailed process of oxidation, the X-ray photoelectron spectroscopy (XPS) was utilized to analyse the elemental composition of MFe2O4 nanoparticles. The Fe 2p3/2 peaks of MFe2O4 were deconvoluted into Fe(Ⅱ) and Fe(Ⅲ) peak (Fig. S6 in Supporting information) [23, 24]. The peak position and mean relative areas of each constituent peak assigned to into Fe(Ⅱ) and Fe(Ⅲ). As shown in the Table 1, the Fe2+:Fe3+ = 0.335:0.665 was got from Fe3O4 for 0 day, while the ratio of Fe2+and Fe3+ gradually became to 0.285:0.715 after preservation for 70 days. Likewise, the same trend could be found in MnFe2O4 nanoparticles, and the Fe2+: Fe3+ = 0.321:0.679 was got from MnFe2O4 for 0 day, after 70 days, the ratio of Fe2+ and Fe3+ turned to 0.265:0.735. Interestingly, the ratio of Fe2+ and Fe3+ in ZnFe2O4 was calculated to be 0.330:0.670 for 0 day, however, the ratio kept nearly stable with 0.321:0.679 for 70 days, demonstrating slight oxidation of this sample. The same method was then used to evaluate the Fe 3p peaks, the same conclusion verified that the ratio of Fe2+ and Fe3+ maintained stable in ZnFe2O4 nanoparticles but decreased in Fe3O4 and MnFe2O4 (Fig. S7 and Table S3 in Supporting information). We hypothesized that ZnFe2O4 nanoparticles owned capacity of anti-oxidation after Zn element partly substituted Fe element in magnetite for Zn only possesses divalent oxidation state. Hence, decline of photothermal resulted from the reduction of NIR absorption and photothermal conversion efficiency, due to the oxidation from Fe2+ to Fe3+, thus the ZnFe2O4 nanoparticles showed a more stable photothermal effect.
|
|
Table 1 The peak position and mean relative areas of the XPS Fe 2p3/2 peaks obtained from MFe2O4 nanoparticles. The table also shows the ratios of Fe2+ and Fe3+ in MFe2O4 nanoparticles. |
Inspired by the stable photothermal effects of ZnFe2O4 nanoparticles, the bio-compatibility was carried out to verify their anticancer performance in vitro. For ZnFe2O4 nanoparticles, the cytotoxicity of normal cells (HEK 293 T) was tested by the standard Cell Counting Kit-8 (CCK-8) assay. The results demonstrated that there was a very little cytotoxicity until the concentration was increased to 1000 μg/mL (Fig. 3a). Then we detected the toxicity in some cancer cell lines (A549, MCF-7 and Hela), the results showed that the cell viability were over 80% (Fig. 3b), even at the highest concentration (1000 μg/mL), indicating an excellent bio-compatibility and safety of ZnFe2O4 nanoparticles. Even stored for 210 days, ZnFe2O4 nanoparticles still showed little cytotoxicity to 293 T cells after being stored for 210 days (Fig. S8c in Supporting information). Subsequently A549 cells were selected as a cell model to investigate photothermal effects in vitro. A549 cells were incubated with ZnFe2O4 nanoparticles and then irradiated by 808 nm laser (3 W/cm2) for different time. About 6.2%, 30.0%, 69.3% of cells were killed by ZnFe2O4 at the concentration of 100 μg/mL for different time of 60, 180, 300 s, respectively (Fig. 3c). The result showed that the cell viability declined with the increasement of illumination time. As irradiation with higher power would damage the irradiated area of skin for clinical therapy, we conducted the following experiment with lower laser power density (2 W/cm2) and longer illumination time. Then the A549 cells were irradiated by 808 nm laser (2 W/cm2) for 600 s after incubating with ZnFe2O4 dispersion at different concentrations. As shown in Fig. 3d, about 26.9% and 78.9% of cells were killed by ZnFe2O4 nanoparticles at the concentration of 100 and 200 mg/mL, respectively, demonstrating that ZnFe2O4 nanoparticles can kill cancer cells under NIR light with a lower power density. Thus we can draw a conclusion that ZnFe2O4 was an excellent and stable photothermal conversion agent and showed significant cancer cell killing ability.

|
Download:
|
| Fig. 3. Relative cell viabilities of (a) 293 T, (b) MCF-7, Hela and A549 cells treated with ZnFe2O4 at different concentrations. (c) Relative cell viabilities of A549 cells treated with ZnFe2O4 at the concentration of 100 μg/mL with NIR laser irradiation (λ = 808 nm, 3 W/cm2) for different time. (d) Relative cell viabilities of A549 cells treated with ZnFe2O4 at different concentrations with or without NIR laser irradiation (λ = 808 nm, 2 W/cm2) for 600 s. | |
In summary, we synthesized three types of ferrites, Fe3O4, MnFe2O4 and ZnFe2O4 nanoparticles. Photothermal effect of Fe3O4 and MnFe2O4 nanoparticles declined owing to oxidation in 70 days storage time. ZnFe2O4 nanoparticles exhibited superior and stable photothermal effect, due to their stronger ability of protecting themselves from being oxidized. Moreover, ZnFe2O4 nanoparticles showed an excellent photothermal effect in vitro. Our research encouraged further exploration of PTAs based on ferrites for application in cancer therapy.
AcknowledgmentsThis work was supported by the National Key R&D Program of China (No. 2016YFC1100300) and National Natural Science Foundation of China (Nos. 51873041 and 51473037).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.005.
| [1] |
R.L. Siegel, K.D. Miller, A. Jemal, CA Cancer J. Clin. 67 (2017) 7-30. DOI:10.3322/caac.21387 |
| [2] |
A. Sudhakar, J. Cancer Sci. Ther. 1 (2009) 1-4. |
| [3] |
L. Cheng, C. Wang, L.Z. Feng, K. Yang, Z. Liu, Chem. Rev. 114 (2014) 10869-10939. DOI:10.1021/cr400532z |
| [4] |
X.H. Huang, I.H. EI-Sayed, W. Qian, M.A. EI-Sayed, J. Am. Chem. Soc. 128 (2006) 2115-2120. DOI:10.1021/ja057254a |
| [5] |
L. Zhang, D.L. Sheng, D. Wang, et al., Theranostics 8 (2018) 1591-1606. DOI:10.7150/thno.22430 |
| [6] |
J. Yu, W.Y. Yin, X.P. Zheng, et al., Theranostics 5 (2015) 931-945. DOI:10.7150/thno.11802 |
| [7] |
D.L. Sheng, T.Z. Liu, L.M. Deng, et al., Biomaterials 165 (2018) 1-13. DOI:10.1016/j.biomaterials.2018.02.041 |
| [8] |
J.B. Qin, Z.Y. Peng, B. Li, et al., Nanoscale 7 (2015) 13991-14001. DOI:10.1039/C5NR02521D |
| [9] |
Y.C. Wang, K.C.L. Black, H. Luehmann, et al., ACS Nano 7 (2013) 2068-2077. DOI:10.1021/nn304332s |
| [10] |
J.G. Piao, L.M. Wang, F. Gao, et al., ACS Nano 8 (2014) 10414-10425. DOI:10.1021/nn503779d |
| [11] |
C. Wang, L.G. Xu, C. Liang, et al., Adv. Mater. 26 (2014) 8154-8162. DOI:10.1002/adma.201402996 |
| [12] |
P.Y. Zhang, H.Y. Huang, J.J. Huang, et al., ACS Appl. Mater. Interfaces 7 (2015) 23278-23290. DOI:10.1021/acsami.5b07510 |
| [13] |
F.F. Zhou, S.N. Wu, S. Song, et al., Biomaterials 33 (2012) 3235-3242. DOI:10.1016/j.biomaterials.2011.12.029 |
| [14] |
N. Lu, P. Huang, W.P. Fan, et al., Biomaterials 126 (2017) 39-48. DOI:10.1016/j.biomaterials.2017.02.025 |
| [15] |
P.F. Zhao, M.B. Zheng, C.X. Yue, et al., Biomaterials 35 (2014) 6037-6046. DOI:10.1016/j.biomaterials.2014.04.019 |
| [16] |
Y.J. Chen, Z.H. Li, H.B. Wang, et al., ACS Appl. Mater. Interfaces 8 (2016) 6852-6858. DOI:10.1021/acsami.6b00251 |
| [17] |
M.X. Zhang, Y.H. Cao, L.N. Wang, et al., ACS Appl. Mater. Interfaces 7 (2015) 4650-4658. DOI:10.1021/am5080453 |
| [18] |
Z.G. Zhou, Y.N. Sun, J.C. Shen, et al., Biomaterials 35 (2014) 7470-7478. DOI:10.1016/j.biomaterials.2014.04.063 |
| [19] |
S. Zanganeh, G. Hutter, R. Spitler, et al., Nature Nanotech. 11 (2016) 986-994. DOI:10.1038/nnano.2016.168 |
| [20] |
Y. Li, X.M. Zhang, C.H. Deng, Chem. Soc. Rev. 42 (2013) 8517-8539. DOI:10.1039/c3cs60156k |
| [21] |
H.B. Peng, S.W. Tang, Y. Tian, et al., Part. Part. Syst. Charact. 33 (2016) 332-340. DOI:10.1002/ppsc.201600071 |
| [22] |
S.H. Xuan, F. Wang, Y.X.J. Wang, J.C. Yu, K.C.F. Leung, J. Mater. Chem. 20 (2010) 5086-5094. DOI:10.1039/c0jm00159g |
| [23] |
H. Deng, X.L. Li, Q. Peng, et al., Angew. Chem. Int. Ed. 44 (2005) 2782-2785. DOI:10.1002/anie.200462551 |
| [24] |
P.C.J. Graat, M.A.J. Somers, Appl. Surf. Sci. 100 (1996) 36-40. |
| [25] |
T. Yamashita, P. Hayes, Appl. Surf. Sci. 254 (2008) 2441-2449. DOI:10.1016/j.apsusc.2007.09.063 |
| [26] |
H. Zhang, L. Li, X.L. Liu, et al., ACS Nano 11 (2017) 3614-3631. DOI:10.1021/acsnano.6b07684 |
| [27] |
S.H. Xuan, Y.X.J. Wang, J.C. Yu, K.C.F. Leung, Chem. Mater. 21 (2009) 5079-5087. DOI:10.1021/cm901618m |
| [28] |
W. Tao, X.Y. Ji, X.D. Xu, et al., Angew. Chem. Int. Ed. 56 (2017) 11896-11900. DOI:10.1002/anie.201703657 |
| [29] |
H.H. Xie, Z.B. Li, Z.B. Sun, et al., Small 12 (2016) 4136-4145. DOI:10.1002/smll.201601050 |
| [30] |
C.Y. Xing, G.H. Jing, X. Liang, et al., Nanoscale 9 (2017) 8096-8101. DOI:10.1039/C7NR00663B |
| [31] |
X.Y. Ji, N. Kong, J.Q. Wang, et al., Adv. Mater. 30 (2018) 1803031. DOI:10.1002/adma.201803031 |
 2019, Vol. 30
2019, Vol. 30