b School of Optoelectronic Science and Engineering, University of Electronic Science and Technology of China(UESTC), Chengdu 610054, China
The field of organic light emitting diodes (OLEDs) has witnessed rapid progress accompanied by successful commercialization in the past few decades due to their unique advantages [1-4]. In order to obtain higher exciton utilization, phosphorescent and thermally activated delayed fluorescence (TADF) emitters have been extensively developed [5-8], which can break through the spin statistics rules and achieve nearly 100% internal quantum efficiency (IQE) [9, 10]. However, the serious efficiency roll-off of blue PhOLEDs and TADF emitters at high luminance usually increases power consumption and reduces the device operating lifetime [11]. Like phosphorescent materials, most TADF materials are also needed to be doped into proper host with high triplet energy [12-14]. This method cannot completely solve the problem of efficiency roll-off, and also increases the difficulty and cost of manufacturing process [15, 16]. Therefore, developing efficient deep blue-emitting fluorescent materials suitable for non-doped device that can achieve high efficiency at high luminescence is a pressing issue toward practical applications, such as display and lighting resource.
Phenanthroimidazole (PI) is widely used in the design of deep blue-emitting fluorescent materials due to its bipolar character and rigid planar π conjugation, which can balance carrier transporting property leading to better luminance efficiency [17-19]. On the other hand, anthracene is also a highly emissive blue emitting moiety, which is often adopted as functional group to construct TTA materials [20, 21]. More importantly, anthracene plays an important role in alleviating efficiency roll-off [19, 20]. Benzonitrile group is incorporated into the molecule due to its electron withdrawing property that can lower the lowest unoccupied molecular orbital (LUMO) energy level and reduce electron injection barrier in device [22, 23]. [1,2,4]Triazolo[1,5-a] pyridine (TP) is a novel building block that is applied to construct the bipolar PhOLED host materials and fluorescent emitter [24-27]. The TP moiety possesses two nitrogen atoms with pyridinelike electron-deficient properties, because the lone electron pair from the nitrogen atoms occupy the hybridized sp2 orbital [28]. The TP behaves as an extended rigid plane to 1, 2, 4-triazole which is essential to form thermal stable films. By combining phenanthroimidazole with anthracene via meta-linked pattern of the benzene ring, it is anticipated that the extent of conjugation could be well confined [29], so that the obtained molecules can possess efficient deep-blue emitting.
In this work, we designed and synthesized two materials PPIAn-CN and PPI-An-TP (Fig. 1). The synthesis and characterization information are provided in the Supporting information. As expected, significant low efficiency roll-off is achieved with about 99% of the maximum external quantum efficiency (EQEmax) maintained even at a high luminance of 1000 cd/cm2 of PPI-AnCN and PPI-An-TP-based non-doped devices. When doping the new materials in CBP (4, 4'-bis(N-carbazolyl)-1, 1'-biphenyl), the doped devices still exhibit excellent stability at high brightness with CIEy ≈ 0.07 and low turn-on voltage of only 2.8 V. The stateof-the-art low efficiency roll-off makes the new materials attractive for commercial applications.

|
Download:
|
| Fig. 1. Molecular structures of PPI-An-CN and PPI-An-TP. | |
The thermal properties of PPI-An-CN and PPI-An-TP were investigated with thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) under a nitrogen atmosphere at a heating rate of 10 C/min, as shown in Fig. 2. Their decomposition temperatures (Td, corresponding to 5% weight loss) were measured to be high to 480 C and 500 C for PPI-An-CN and PPI-An-TP, respectively. Owing to the bulky and rigid skeleton of the TP unit, PPI-An-TP has a higher decomposition temperature. Additionally, the glass-transition temperature of PPI-An-CN is 197 C, while no obvious glass transition and an endothermic melting transition could be observed for PPI-An-TP. These results demonstrate that the two molecules are stable enough for thermal evaporation and device operation, which will positively alleviate the device's efficiency roll-off. The detailed data are summarized in Table 1.

|
Download:
|
| Fig. 2. TGA and DSC curves of PPI-An-CN and PPI-An-TP. | |
|
|
Table 1 The thermal and photophysical properties of PPI-An-CN and PPI-An-TP. |
UV-vis absorption and PL spectra of PPI-An-CN and PPI-An-TP in diluted dichloromethane solution (≈ 106 mol/L) and film are shown in Fig. 3 (Table 1). The two compounds show similar absorption bands. The absorption band at approximately 258 nm may originate from the π-π* transition of two biphenyl units connected with imidazole. The longer wavelength absorption bands at 330 nm, which may originate from the π-π* transitions of the PI group. A weak peak at about 400 nm was observed, which should be assigned to the π-π* transition of the anthracene group. The optical energy gap can be estimated from the absorption onset to be 2.96 eV of PPI-An-CN and 3.01 eV of PPI-An-TP. For the emission spectra, it should be noted that meta-linked pattern of the benzene ring can efficiently suppress p-conjugation. The two compounds exhibit strong deep blue emissions at 436 nm and 430 nm in CH2Cl2 with high photoluminescent quantum yield (PLQY) of 85% and 88%. Moreover, redshift of 8 nm (PPI-An-CN) and 7 nm (PPI-An-TP) is observed in the solid thin film compared with those in solution (Figs. 3a and b), indicating the interaction of π-π stacking on emission in the solid is effectively suppressed.

|
Download:
|
| Fig. 3. Absorption and PL spectra of PPI-An-CN (a) and PPI-An-TP (b) in DCM and solid state; PL spectra of PPI-An-CN (c) and PPI-An-TP (d) in different solvents. | |
To study the relationship between the molecular structure and luminescence properties of PPI-An-CN and PPI-An-TP, we studied their PL spectra in solvents with different polarities. As can be seen from Figs. 3c and d, as the solvent polarity increased from lowpolarity toluene to high-polarity acetonitrile, the emission peak position was nearly unchanged, indicating that the intramolecular charge transfer (ICT) effect was well controlled and suppressed the influence of carrier transporting moieties on the chromophores. This is mainly due to the meta-linked pattern of the molecule, which effectively suppressed the molecular conjugation, resulting in negligible intramolecular charge transfer (ICT) effect.
Cyclic voltammetry (CV) was conducted to calculate the highest occupied molecular orbital (HOMO) levels of PPI-An-CN and PPI-AnTP. The HOMO levels were measured to be 5.63 and 5.58 eV (Table 1 and Fig. S4 in Supporting information), the lowest unoccupied molecular orbital (LUMO) levels were further projected to be 2.67 eV for PPI-An-CN and 2.57 eV for PPI-An-TP. Clearly, the electron injection ability of PPI-An-CN and PPI-An-TP is similar to thatof 1, 3, 5-tri(phenyl-2-benzimidazolyl)-benzene (TPBi, 2.70 eV), but better than that of phenanthroimidazole (2.16 eV) [17, 30]. This suggests a good carrier injection property in PPI-An-CN and PPIAn-TP, due to their suitable HOMO and LUMO energy levels. This property allowed the fabrication of devices with simple configurations, which is an advantage for practical OLED applications.
To further understand the different energy gaps obtained from absorption spectra and CV measurements, density functional theory (DFT) calculations were carried out at a level of vB97XD/6– 311 G(d), employing the Gaussian 16A package. Orbitals were analyzed using Multiwfn program (version 3.6) [31]. The geometries, molecular orbitals (MOs) and calculated energy levels are shown in Fig. 4. The HOMO of PPI-An-CN was mainly located on the PI unit while the LUMO was mainly located on the anthracene unit. It shows complete separation between HOMO and LUMO. After converting the cyanophenyl section to TP moiety, the distribution of the HOMOs was transferred into the anthracene unit owing to the weaker electron-withdrawing of TP moiety. And the lower LUMO values of PPI-An-CN agreed with the results of the electrochemical measurements. Interestingly, the HOMO-1 levels of PPI-An-CN were located mostly on the anthracene moiety, similar to the HOMOs in PPI-An-TP. The calculated energy gap from the HOMO-1 to the LUMO was 6.85 eV for PPI-An-CN, which was close to the energy gap from HOMO to LUMO in PPI-An-TP (6.84 eV). Exactly, these results were consistent with the optical bandgaps obtained from the absorption spectra, confirming the hypothesized separation of the electrical and optical energy gaps. In fact, the transition from HOMO-1 to LUMO and HOMO to LUMO + 1 in PPI-An-CN and from HOMO-1 to LUMO + 1 in PPI-AnTP were allowed because of their effective overlaps in the MOs. However, the transitions from the HOMO to the LUMO in the PPIAn-CN was nearly forbidden in the photo-absorption process, owing to the complete separation characters.
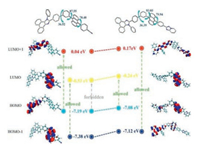
|
Download:
|
| Fig. 4. Relevant molecular orbital amplitude plots, energy levels and possible transition sketch maps of PPI-An-CN and PPI-An-TP. | |
In order to examine the excited state properties of the compounds, hole-electron analysis for the S0-Sn and Tn (n = 1–5) transitions were performed using Multiwfn program (version 3.6) based on time-dependent density functional theory (TD-DFT) (Figs. S5 and S6 in Supporting information). Both two molecules exhibit significant LE characteristics. To describe the fragment contribution to hole and electron of PPI-An-CN and PPI-An-TP accurately, we artificially divided the molecule into four fragments. As shown in Fig. 5, for the S0-S1 transition in PPI-An-CN, the "hole" and "electron" were mostly located on the anthracene moiety. This distribution was assigned to the LE transition, and the large overlap in anthracene moiety implied that the compound would have high fluorescence efficiency. The homologous LE character of the PI moiety was also observed in the S0-S3 transition. PPI-An-TP showed similar excited state properties. We speculate that the meta-linked pattern of the benzene ring in fragment 2 greatly inhibits electron withdrawing-donating property, so that the electron-withdrawing group in fragment 4 has little influence on the excited state properties. Importantly, the oscillator strength of S0-S1 that was considered to be related to the PLQYs of the emitters was enhanced upon converting the cyanophenyl moiety to TP moiety, with values of 0.3562 for PPI-An-CN, 0.4147 for PPI-An-TP. The obvious increase in the oscillator strength was in good agreement with the PLQYs in the experimental measurements. The homologous LE character of the PI moiety and anthracene moiety plays a vital role in alleviating efficiency roll-off [19, 20].

|
Download:
|
| Fig. 5. Fragment contribution to hole and electron of PPI-An-CN and PPI-An-TP. | |
To evaluate electrical properties of PPI-An-CN and PPI-An-TP, hole- and electron-only devices were fabricated with the respective configuration of ITO/NPB (10 nm)/PPI-An-CN or PPI-An-TP (80 nm)/NPB (10 nm)/Al (100 nm) and ITO/TPBI (10 nm)/PPI-AnCN or PPI-An-TP (80 nm)/TPBI (10 nm)/LiF (1 nm)/Al(100 nm). As shown in Fig. 6, both hole-only and electron-only devices exhibit high current densities in the voltage range typically suitable for OLEDs, indicating that these two materials have both hole-transporting and electron-transporting abilities and possess a bipolar carrier transport feature. Meanwhile, the hole and electron currents of PPI-An-TP are more balanced than PPI-An-CN, which can be explained by introducing TP group with better electron transporting capacity and the relatively deep LUMO level. Such bipolar nature is conducive to delivering high efficiency when PPIAn-CN and PPI-An-TP are used as emitters.
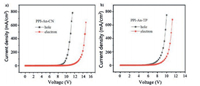
|
Download:
|
| Fig. 6. Current density versus voltage characteristics of the hole-only and electron-only devices for PPI-An-CN (a) and PPI-An-TP (b). | |
Non-doped OLED devices (M) were fabricated with a simple configuration of ITO/MoO3 (3 nm)/TAPC (70 nm)/TCTA (10 nm)/ PPI-An-CN or PPI-An-TP (20 nm)/TPBi (40 nm)/LiF (0.8 nm)/Al (80 nm). In this device, PPI-An-CN and PPI-An-TP function as a blue emitting layer (EML). TAPC is used as the hole-transporting layer (HTL) and TCTA is a buffer layer, TPBI is the electrontransporting layer (ETL) and hole-blocking layer, LiF is electron injection layer. The EL spectrum (Fig. 7a) peaked at 444 nm for the PPI-An-CN device and 439 nm for the PPI-An-TP device, and the CIE coordinates were (0.15, 0.11) for PPI-An-CN and (0.15, 0.09) for PPIAn-TP. Fig. 7b shows the luminescence -voltage- current density curves, which indicate that both devices have very low turn-on voltages (3 V for PPI-An-CN and PPI-An-TP), revealing the small energy gap in the devices. As shown in Figs. 7c and 3d, the PPI-An-CN based non-doped device showed a maximum external quantum efficiency (EQE), current efficiency (CE) and power efficiency (PE) of 3.28%, 3.25 cd/A and 2.20 lm/W, respectively. The PPI-An-TP non-doped device also exhibited good performance, exhibiting a maximum EQE, CE and PE of 3.11%, 3.15 cd/A and 2.68 lm/W, respectively. The reasonable device structure plays an important role in alleviating efficiency roll-off. The PPI-An-CN and PPI-An-TP-based non-doped devices exhibited negligible EQE rolloff of 0.61% and 1.61% at a high luminance of 1000 cd/cm2. The key data of the devices are summarized in Table 2.

|
Download:
|
| Fig. 7. (a) EL spectrum; (b) Luminescence -voltage- current density characteristic curves; (c) plots of current efficiency and power efficiency; (d) EQE of the PPI-An-CN- and PPI-An-TP-based non-doped devices. | |
|
|
Table 2 Key performance summary of the PPI-An-CN- and PPI-An-TP-based non-doped and doped devices. |
To further improve the color purity and device efficiency, the doped OLEDs were constructed with a multilayer structure (D): ITO/MoO3 (3 nm)/TAPC (70 nm)/TCTA (10 nm)/ CBP : 40 wt% PPIAn-CN or PPI-An-TP (20 nm)/TPBi (40 nm)/LiF (0.8 nm)/Al (80 nm). Here, CPB was selected as the host. The energy level diagrams and molecular structures of the materials used in these devices are depicted in Fig. 8. The PPI-An-CN-doped devices revealed blue emission peaks of 439 nm and CIE coordinates of (0.15, 0.08). The PPI-An-TP-doped devices revealed blue emission peaks of 434 nm and CIE coordinates of (0.15, 0.07). The turn-on voltages of the two devices were only 2.8 V. The PPI-An-CN-based doped device exhibited a maximum EQE, CE and PE of 3.79%, 2.63 cd/A and 2.50 lm/W, respectively. The device using PPI-An-TP as a deep-blue emitter exhibited impressive performance, with a maximum EQE, CE and PE of 3.57%, 2.39 cd/A and 2.27 lm/W, respectively. As shown in Fig. 9d, the doped devices' EQE still exhibited excellent stability at high brightness. The device characteristics are shown in Fig. 9 and Table 2.
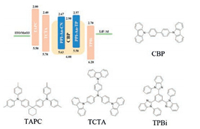
|
Download:
|
| Fig. 8. Schematic energy level diagram and the molecular structures of the used materials in doped devices. | |

|
Download:
|
| Fig. 9. (a) EL spectrum; (b) Luminescence-voltage-current density characteristic curves; (c) plots of current efficiency and power efficiency; (d) EQE of the PPI-An-CN- and PPI-An-TP-based doped device. | |
In summary, two efficient blue-emitting molecules PPI-An-CN and PPI-An-TP, which consist of PI with benzonitrile substituted anthracene or TP substituted anthracene via meta-linked pattern of the benzene ring, are designed and synthesized. From the theoretical computation and photophysical properties, we have demonstrated that PPI-An-CN and PPI-An-TP have LE features in PI and anthracene moiety. By using PPI-An-CN as emitter in a nondoped device, high EQE of 3.25% and slightly efficiency roll-off of 0.6% at a high luminance of 1000 cd/cm2 are obtained. Particularly, the PPI-An-TP-based doped device also exhibits high EQE of 3.57% and low efficiency roll-off with CIEy≈0.07 and low turn-on voltage of only 2.8 V. The TP moiety exhibits superior electron transporting capacity and is beneficial for deep blue emitting, which deserves further study.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 51673113) and the Key Project of DEGP (No. 2018KZDXM032).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.09.013.
| [1] |
H. Sasabe, J. Kido, J. Mater. Chem. C 1 (2013) 1699-1707. DOI:10.1039/c2tc00584k |
| [2] |
Y. Sun, N.C. Giebink, H. Kanno, et al., Nature 440 (2006) 908-912. DOI:10.1038/nature04645 |
| [3] |
C.W. Tang, S.A. VanSlyke, Appl. Phys. Lett. 51 (1987) 913-915. DOI:10.1063/1.98799 |
| [4] |
H. Uoyama, K. Goushi, K. Shizu, H. Nomura, C. Adachi, Nature 492 (2012) 234-238. DOI:10.1038/nature11687 |
| [5] |
Y.H. Zhu, Y.K. Yang, Y.J. Wang, et al., Adv. Opt. Mater. 6 (2018) 1701320.
|
| [6] |
Y.K. Yang, S.M. Wang, Y.H. Zhu, et al., Adv. Funct. Mater. 28 (2018) 1706916.
|
| [7] |
S.P. Wang, Y. Miao, X.J. Yan, K.Q. Ye, Y. Wang, et al., J. Mater. Chem. C 6 (2018) 6698-6704. DOI:10.1039/C8TC01746H |
| [8] |
X. Yang, G. Zhou, W.Y. Wong, et al., Chem. Soc. Rev. 44 (2015) 8484-8575. DOI:10.1039/C5CS00424A |
| [9] |
Y. Ma, H. Zhang, J. Shen, C. Che, et al., Synthetic Met. 94 (1998) 245-248. DOI:10.1016/S0379-6779(97)04166-0 |
| [10] |
M.A. Baldo, D.F. O'Brien, Y. You, et al., Nature 395 (1998) 151-154. DOI:10.1038/25954 |
| [11] |
C. Xiang, X. Fu, W. Wei, et al., Adv. Funct. Mater. 26 (2016) 1463-1469. DOI:10.1002/adfm.201504357 |
| [12] |
X. Chen, X.M. Zhuang, Z.Y. Wang, et al., Org. Electron. 69 (2019) 85-91. DOI:10.1016/j.orgel.2019.03.013 |
| [13] |
L. Zhang, K.W. Cheah, et al., Sci. Rep-Uk 8 (2018) 8832.
|
| [14] |
W.C. Chen, Y. Yuan, Z.L. Zhu, et al., Chem. Sci. 9 (2018) 4062-4070. DOI:10.1039/C8SC00282G |
| [15] |
W. Li, J. Zhao, L. Li, et al., Org. Electron. 58 (2018) 276-282. DOI:10.1016/j.orgel.2018.04.027 |
| [16] |
C.W. Lee, J.Y. Lee, et al., Chem. Commun. (Camb.) 49 (2013) 1446-1448. DOI:10.1039/c2cc38049h |
| [17] |
Z.L. Zhu, S.F. Ni, W.C. Chen, et al., J. Mater. Chem. C 6 (2018) 3584-3592. DOI:10.1039/C7TC04972B |
| [18] |
Z.Y. Wang, B. Liu, J.W. Zhao, et al., Org. Electron. 52 (2018) 89-97. DOI:10.1016/j.orgel.2017.09.051 |
| [19] |
X.Y. Tang, Q. Bai, T. Shan, et al., Adv. Funct. Mater. 28 (2018) 1705813.
|
| [20] |
Y. Yu, L. Ma, X.L. Yang, et al., Adv. Opt. Mater. 6 (2018) 180060.
|
| [21] |
C. He, H. Guo, Q. Peng, S. Dong, F. Li, et al., J. Mater. Chem. C 3 (2015) 9942-9947. DOI:10.1039/C5TC02055G |
| [22] |
S.W. Li, C.H. Yu, C.L. Ko, et al., ACS Appl. Mater. Inter. 10 (2018) 12930-12936. DOI:10.1021/acsami.8b02766 |
| [23] |
X. Cao, D. Zhang, S. Zhang, Y. Tao, W. Huang, et al., J. Mater. Chem. C 5 (2017) 7699-7714. DOI:10.1039/C7TC02481A |
| [24] |
W. Song, L. Gao, T. Zhang, J. Huang, J. Su, J. Lumin. 206 (2019) 386-392. DOI:10.1016/j.jlumin.2018.09.006 |
| [25] |
W.X. Song, Y. Chen, Q.H. Xu, et al., ACS Appl. Mater. Inter. 10 (2018) 24689-24698. DOI:10.1021/acsami.8b07462 |
| [26] |
W. Song, L. Shi, L. Gao, et al., ACS Appl. Mater. Inter. 10 (2018) 5714-5722. DOI:10.1021/acsami.7b18202 |
| [27] |
C. Cao, W.C. Chen, S. Tian, et al., Mat. Chem. Front. 3 (2019) 1071-1079. DOI:10.1039/C8QM00678D |
| [28] |
W.C. Chen, Y. Yuan, S.F. Ni, et al., ACS Appl. Mater. Inter. 9 (2017) 7331-7338. DOI:10.1021/acsami.6b14638 |
| [29] |
J. Huang, N. Sun, Y. Dong, et al., Adv. Funct. Mater. 23 (2013) 2329-2337. DOI:10.1002/adfm.201202639 |
| [30] |
Z. Wang, P. Lu, S. Chen, et al., J. Mater. Chem. 21 (2011) 5451-5456. DOI:10.1039/c1jm10321k |
| [31] |
T. Lu, F.W. Chen, J. Comput. Chem. 33 (2012) 580-592. DOI:10.1002/jcc.22885 |
 2019, Vol. 30
2019, Vol. 30 



