b Frontier Institute of Science and Technology, State Key Laboratory for Strength and Vibration of Mechanical Structures, Xi'an Jiaotong University, Xi'an 710054, China
Picric acid, 2, 4, 6-trinitrophenol (PA) [1], is a strong organic acid, which was commonly used in industry, pharmaceuticals, and chemical laboratories [2]. However, PA is very highly irritant and an allergen, which has made it an important environmental pollutant, and its sensitive detection has attracted more concern recently [3]. More importantly, PA is a typical polynitrated aromatic compound, which is a powerful explosive [4]. Therefore, the development of efficient sensors to detect PA at very low concentration in order to prevent terrorist threats as well as environmental pollution is a very appealing field of research [5, 6]. Till now, many methods have been employed for the detection of nitroaromatic explosives, especially for PA. The fluorescent sensor was considered as a very efficient strategy to detect nitroaromatic explosives among another methods, due to the high sensitivity, reversibility and easy sample preparation, etc. [7-13]. Although lots of fluorescent sensing system have been developed to detect nitroaromatic explosives, development of more efficient organic chemosensors with high selectivity for PA is still challenging [14-18].
Considering the dominant position of picrate anions in aqueous solutions, the introduction of the cationic group into the organic fluorophore should be beneficial to the sensing performance. Mukherjee and coworkers synthesized two anthracene-functionalized fluorescent tris-imidazolium salts, which showed excellent sensing performance of PA at the ppb level in both organic and aqueous media [19]. Fang and coworkers developed a sensing film for PA with high selectivity by the combination of the hexaphenylsilole (HPS) nanoparticles and chitosan. The selectivity of the film was attributed to the specific electrostatic association effect of the protonated substrate film to picrate anion [20]. These works inspired us to introduce cationic group to the widely used conjugated system to develop novel fluorescent molecules for the detection of PA with good performance.
Viologens were di-quaternized 4, 4'-bipyridyl salts, which have been studied during past several decades [21-24]. The cationic pyridine salts not only gave the viologen derivatives excellent redox properties, but also made these compounds soluble in aqueous media [25]. However, the viologens were non-emission due to the electron-accepting capability, which hindered their development [26, 27]. In order to enhance the emission of the viologen derivatives, many conjugated scaffolds were introduced into the extension of the π-conjugation between the two pyridinium units. Many fluorescent viologen derivatives, including thiazolo[5, 4-d]thiazole viologen [28], thienoviologen [17], 1, 4-bis-(4-pyridylethynyl)benzene viologen [29], [5]heli-viologens [30] were synthesized (Fig. 1). Pyrene is a typical conjugated polycyclic aromatic compound with high fluorescence quantum yield [31-33], which has been widely used as building blocks to develop highly emissive conjugated molecules and polymers for nitroaromatic explosives through the electron transfer between them via electron donation and acceptation interaction [34, 35]. The attempts have been made to combine pyrene and viologens by many research groups, however, the charge transfer between an electron donor (pyrene) and an electron acceptor (viologen) made the viologen derivatives non-emissive [36, 37]. However, introducing pyrene into the viologen system should significantly enhance the emission properties of novel viologen derivatives. Actually, the pyrenoviolgens, 1, 3, 6, 8-tetrakis(N-methylpyridinium-4-yl)pyrene (Py4+) with impressive fluorescence properties (i.e., Φ = 70% and τ = 3.4 ns) was synthesized by Takagi group in 2012 [38], which was also widely used in color tuning and emission amplification [39-41]. However, pyrenoviologens has never been used in the detection of detect nitro-aromatic compounds.

|
Download:
|
| Fig. 1. Selected examples of emissive viologen derivatives. | |
Based on these considerations, it can be envisioned that the cationic pyrenoviologens may be used to fabricate fluorescent sensors for the detection of the PA in aqueous solution. The cationic nature of pyrenoviologens will offer superior sensitivity and selectivity. Thus, in this contribution, the pyrenoviologen derivative, 1, 6-di(N-methylpyridinium-4-yl)pyrene (Py2+) viologen as well as 1, 3, 6, 8-tetrakis(N-methylpyridinium-4-yl)pyrene (Py4+) were used to detect PA in aqueous media. The results showed that the sensors are highly sensitive, and can identify the PA from other nitro-aromatic compounds.
The pyridine precursors, 2 and 5 were synthesized by Suzuki coupling reaction between starting material 1 or 4 and 4-pyridinyl boronic acid, using Pd(PPh3)4 as a catalyst and Aliquant-336 as a phase-transfer agent (Scheme 1). Compound 2 was obtained in 60% yield as a yellow powder. The previously known compound, 5 was obtained as a yellow powder, which was consistent with the previously reported data [38]. The pyridine derivatives, 2 and 5 were respectively converted into the 1, 6-di(N-methylpyridinium-4-yl)pyrene (Py2+, 3) viologen and 1, 3, 6, 8-tetrakis(N-methylpyridinium-4-yl)pyrene (Py4+, 6) via reaction with iodomethane (MeI), which were obtained as a solid (3: off-white, 6: dark green, which was reported previously [22]) in a high yield of ca. 80%. The pyrenoviologens, 3 and 6 were characterized by NMR, melting point and high-resolution mass spectrometry (HRMS).

|
Download:
|
| Scheme 1. Synthesis of pyrenoviologen derivatives 3 and 6. | |
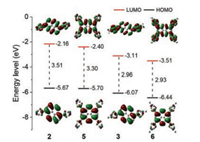
The pyrenoviologens, 3 and 6 showed good optical properties in aqueous media. Fig. 2a illustrates the UV–vis spectra of 3 and 6, which is clear that the significantly bathochromic shift of 6 was attributed to the extending of π-conjugation (the bandgaps of 3 and 6 were 2.83 eV and 2.75 eV, respectively, inset of Fig. 2a). Comparing the profiles of the UV–vis and excitation spectra of 3 and 6 in aqueous media (Figs. S3 and S4 in Supporting information), it is clearly seen that the red edges of 6 was redshifted if compared with that of 3, which also gave strong evidence to support that introduction of pyrene into the conjugated polymer chain did enhance the conjugation. The calculated absorption spectra were well consistent of the experimental data (Figs. S1 and S2 in Supporting information). The DFT calculation results showed that the pyrenoviologens have lower LUMO levels (LUMO: -3.11 eV for 3; -3.51 eV for 6) than the pyridine precursors (Fig. 3). Usually, expanding the π-conjugated systems will significantly decrease the bandgap of molecules, which will give emission red-shifted of the molecules. As shown in Figs. 2 and 3, the bandgap of 6 is smaller than that of 3. However, the maximum emission of 3 was observed at λem = 513 nm, and the maximum emission of 6 was observed at λem = 485 nm (Fig. 2b). Clearly, the emission of 6 was blue-shifted compared with 3 rather than red-shift in aqueous solutions. The quantum yields (QY) of 3 and 6 in water were 64% and 95%, respectively. The abnormal phenomenon was attributed to the twisted intramolecular charge transfer (TICT) properties of viologen derivatives [42-47]. The excellent emission properties provide good benefit to fabricate new optical sensors.

|
Download:
|
| Fig. 2. (a) UV–vis spectra of 3 and 6 in water. Inset shows the extrapolated optical band gaps (Eg). (b) Emission spectra of 3 and 6 in water [3 or 6] = 10 μmol/L. λex = 365 nm. | |
|
Download:
|
| Fig. 3. Comparison of HOMO/LUMO plots for 2, 3, 5 and 6. | |
Consideration of the special structures of pyrenoviologens, 3 and 6, as well as the good emission properties, compounds 3 and 6 were used to fabricate optical sensors for the detection of PA. Fig. 4 depicts the fluorescence emission spectra of 3 at various PA concentrations in an aqueous medium. Compound 3 exhibited an emission maximum at 513 nm, while the emission maximum gradually decreased upon addition of aliquots of PA (Fig. 4a). It can be seen that the fluorescence emission was almost completely quenched when the concentration of PA reached 55 umol/L, with the QY less than 1%. The compound 6 also exhibited almost identical titration features, for the fluorescence spectra, a similar phenomenon was observed, the emission of compound 6 (lem = 482 nm) was gradually quenched after addition of aliquots of PA, and the QY became 15% (Fig. 4b).

|
Download:
|
| Fig. 4. Fluorescence emission spectra of 3 and 6 in the presence of different concentrations of PA in an aqueous medium (λex = 365 nm). | |
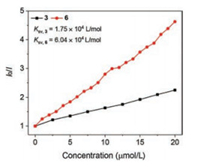
The fluorescence quenching results can be also treated with the Stern–Volmer equation (Fig. 5), I0/I = 1 + Ksv [PA], where I0 and I are the fluorescence intensity of the 3 or 6 in the absence and presence of PA, respectively, and Ksv is the Stern–Volmer constant. In contrast to 3 (Ksv, 3 = 1.75×104 L/mol), the Stern–Volmer plots of 6 showed more efficient fluorescence quenching with much higher quenching constant (Ksv, 6 = 6.04×104 L/mol). The enhanced quenching efficiency of the 6 was likely due to the larger conjugate chain to lower energy quenching sites that were generated upon PA binding.
|
Download:
|
| Fig. 5. Quenching efficiencies of 3 and 6 in the presence of different concentrations of PA. | |
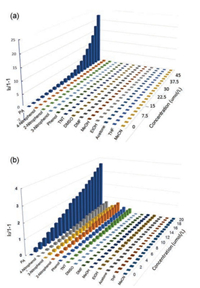
Selectivity is very important for the real-life application of sensors. It is of interest to study the response of the pyrenoviologen-based fluorescent sensor to commonly found explosives and chemicals which may affect the detection of PA in the aqueous phase. Fig. 6 showed the histograms of (I0/I)-1 to the different concentrations of PA and some common interferents. Specifically, upon the addition of PA to the solution of 3 and 6, the fluorescence spectra showed distinct changes in intensity, while 4-nitrophenol, 3-nitrophenol, 2-nitrophenol, phenol, TNT, DMSO, DMF, MeOH, EtOH, acetone, THF, MeCN, and other common solvents have little effect, indicating that the compound 3 and 6 are highly selective to PA.
|
Download:
|
| Fig. 6. Quenching efficiencies of PA and common interferents to the emission of the 3 (a) and 6 (b) at different concentrations. | |
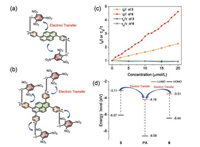
The good sensing performance of pyrenoviologens to PA made us study the mechanism behind them. Firstly, the fluorescence lifetimes of 3 and 6 were determined in the presence of different concentrations of PA, and the results are shown in Fig. 7c. The results showed that the lifetime curves were close to a straight line after adding PA, indicating the static quenching nature of the sensing process. The special selectivity of 3 and 6 to PA may be only understood by the special electrostatic interaction, which were visualized as cartoon in Figs. 7a and b. It is well known that PA behaves as a strong acid because of the three nitro-groups in the molecules. The electron positive pyrenoviologens possessed inherent properties to affinity the electron negative PA in aqueous solutions, which were further confirmed by the 1H NMR titration results and the sensing performances in different pH values (Figs. S5–S8 in Supporting information) [20]. Other structuresimilar compounds, such as 2-nitrophenol, 3-nitrophenol or 4-nitrophenol, showed little quenching efficiency due to the lower acid properties. Other hydrophobic nitroaromatics, such as TNT or commonly used solvents, have no tendency to the hydrophilic pyrenoviologens, resulting in no quenching was observed. The better sensing performance of 6 may be attributed to its lower LUMO level [48], which fascinating the electron transfer between 6 and PA molecule (Fig. 7d).
|
Download:
|
| Fig. 7. Schematic representation of the electron-transfer mechanism for the quenching of the fluorescence of (a) 3 and (b) 6 by PA. (c) Energy levels of HOMO (π) and LUMO (π*) orbitals of 3, 6, PA showing favorable electron transfer from 3 and 6 to the photo-excited state of PA. | |
In conclusion, two highly emissive pyrenoviologen derivatives were synthesized. The pyrenoviologen derivatives, 3 and 6 showed good fluorescence properties. The pyrenoviologen derivatives, 3 and 6 were used to fabricate fluorescent sensors for the detection of picric acid (PA) with good sensitivity (Ksv, 3 = 1.75×104 L/mol; Ksv, 6 = 6.04×104 L/mol). The selectivity of the sensor was attributed to the specific electrostatic association effect of the cationic pyrenoviologens to the picrate anions, which also gave the sensor special selectivity. Fluorescence lifetime measurements revealed that the sensing process was a static quenching. The electron transfer between them was attributed to the fluorescence quenching. The novel sensors and their mechanism offered a new strategy to fabricate the real-life PA sensor.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21603016, 21704081, 51603016 and 21704005), Shaanxi College Students Innovation and Entrepreneurship Training Program (No. S201910710282). We thank Dr. Gang Chang and Yu Wang at Instrument Analysis Center of Xi'an Jiaotong University for their assistance with acquiring PL spectra.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.09.039.
| [1] |
G.V. Perez, A.L. Perez, J. Chem. Educ. 77 (2000) 910.
|
| [2] |
B. Roy, A.K. Bar, B. Gole, P.S. Mukherjee, J. Org. Chem. 78 (2013) 1306-1310. DOI:10.1021/jo302585a |
| [3] |
H. Sohn, R.M. Calhoun, M.J. Sailor, W.C. Trogler, Angew. Chem. Int. Ed. 40 (2001) 2104-2105. DOI:10.1002/1521-3773(20010601)40:11<2104::AID-ANIE2104>3.0.CO;2-%23 |
| [4] |
T. Feng, X. Li, J. Wu, C. He, C. Duan, Chin. Chem. Lett. (2019), doi: http://dx.doi.org/10.1016/j.cclet.2019.04.059.
|
| [5] |
M. Cameron, Picric Acid Hazards, American Industrial Hygiene Association, Fairfax, VA, 1995.
|
| [6] |
M. Nipper, Y. Qian, R. Scott Carr, K. Miller, Chemosphere 56 (2004) 519-530. DOI:10.1016/j.chemosphere.2004.04.039 |
| [7] |
M.E. Germain, M.J. Knapp, Chem. Soc. Rev. 38 (2009) 2543-2555. DOI:10.1039/b809631g |
| [8] |
X. Sun, Y. Wang, Y. Lei, Chem. Soc. Rev. 44 (2015) 8019-8061. DOI:10.1039/C5CS00496A |
| [9] |
S. Rochat, T.M. Swager, ACS Appl. Mater. Interfaces 5 (2013) 4488-4502. DOI:10.1021/am400939w |
| [10] |
U.H.F. Bunz, K. Seehafer, M. Bender, M. Porz, Chem. Soc. Rev. 44 (2015) 4322-4336. DOI:10.1039/C4CS00267A |
| [11] |
W.M. Wan, D. Tian, Y.N. Jing, et al., Angew. Chem. Int. Ed. 57 (2018) 15510-15516. DOI:10.1002/anie.201809844 |
| [12] |
W. Zhang, D. Yu, Z. Wang, et al., Org. Lett. 21 (2019) 109-113. DOI:10.1021/acs.orglett.8b03538 |
| [13] |
W. Zhang, G. Li, L. Xu, et al., Chem. Sci. 9 (2018) 4444-4450. DOI:10.1039/C8SC00688A |
| [14] |
K.M. Hindi, M.J. Panzner, C.A. Tessier, C.L. Cannon, W.J. Youngs, Chem. Rev. 109 (2009) 3859-3884. DOI:10.1021/cr800500u |
| [15] |
L. Ding, Y. Fang, Chem. Soc. Rev. 39 (2010) 4258-4273. DOI:10.1039/c003028g |
| [16] |
S.S. Nagarkar, A.V. Desai, S.K. Ghosh, CrystEngComm 18 (2016) 2994-3007. DOI:10.1039/C6CE00244G |
| [17] |
A. Beneduci, S. Cospito, M. La Deda, G. Chidichimo, Adv. Funct. Mater. 25 (2015) 1240-1247. DOI:10.1002/adfm.201403611 |
| [18] |
X. Wang, N. Gan, M. Gu, et al., Chin. Chem. Lett. (2018), doi: http://dx.doi.org/10.1016/j.cclet.2018.12.023.
|
| [19] |
A. Chowdhury, P.S. Mukherjee, J. Org. Chem. 80 (2015) 4064-4075. DOI:10.1021/acs.joc.5b00348 |
| [20] |
G. He, H. Peng, T. Liu, et al., J. Mater. Chem. 19 (2009) 7347-7353. DOI:10.1039/b906946a |
| [21] |
L. Striepe, T. Baumgartner, Chem. -Eur. J. 23 (2017) 16924-16940. DOI:10.1002/chem.201703348 |
| [22] |
S.L. Li, M. Han, Y. Zhang, et al., J. Am. Chem. Soc. 141 (2019) 12663-12672. DOI:10.1021/jacs.9b04930 |
| [23] |
K. Murugavel, Polym. Chem. 5 (2014) 5873-5884. DOI:10.1039/C4PY00718B |
| [24] |
H. Chen, H. Yang, W.C. Xu, Y.B. Tan, Chin. Chem. Lett. 24 (2013) 857-860. DOI:10.1016/j.cclet.2013.05.028 |
| [25] |
T.T. Cao, X.Y. Yao, J. Zhang, Q.C. Wang, X. Ma, Chin. Chem. Lett. 26 (2015) 867-871. DOI:10.1016/j.cclet.2015.01.032 |
| [26] |
G. Li, L. Xu, W. Zhang, et al., Angew. Chem. Int. Ed. 57 (2018) 4897-4901. DOI:10.1002/anie.201711761 |
| [27] |
G. Li, B. Zhang, J. Wang, et al., Angew. Chem. Int. Ed. 58 (2019) 8468-8473. DOI:10.1002/anie.201903152 |
| [28] |
A.N. Woodward, J.M. Kolesar, S.R. Hall, et al., J. Am. Chem. Soc. 139 (2017) 8467-8473. DOI:10.1021/jacs.7b01005 |
| [29] |
Z. Wang, W. Bai, J. Tong, et al., Chem. Commun. 52 (2016) 10365-10368. DOI:10.1039/C6CC02851A |
| [30] |
X. Zhang, E.L. Clennan, N. Arulsamy, R. Weber, J. Weber, J. Org. Chem. 81 (2016) 5474-5486. DOI:10.1021/acs.joc.6b00835 |
| [31] |
N. Yan, Z. Xu, K.K. Diehn, et al., J. Am. Chem. Soc. 135 (2013) 8989-8999. DOI:10.1021/ja402560n |
| [32] |
N. Yan, Z. Xu, K.K. Diehn, et al., Langmuir 29 (2013) 793-805. DOI:10.1021/la304957n |
| [33] |
Q. Hu, C. Qin, L. Huang, et al., Dyes Pigment. 149 (2018) 253-260. DOI:10.1016/j.dyepig.2017.10.002 |
| [34] |
S. Zhang, F. Lü, L. Gao, L. Ding, Y. Fang, Langmuir 23 (2007) 1584-1590. DOI:10.1021/la062773s |
| [35] |
G. He, N. Yan, J. Yang, et al., Macromolecules 44 (2011) 4759-4766. DOI:10.1021/ma200953s |
| [36] |
M. Hariharan, J. Joseph, D. Ramaiah, J. Phys. Chem. B 110 (2006) 24678-24686. DOI:10.1021/jp063079o |
| [37] |
M. Hariharan, S.C. Karunakaran, D. Ramaiah, I. Schulz, B. Epe, Chem. Commun. 46 (2010) 2064-2066. DOI:10.1039/b924943e |
| [38] |
S. Hagiwara, Y. Ishida, D. Masui, T. Shimada, S. Takagi, Tetrahedron Lett. 53 (2012) 5800-5802. DOI:10.1016/j.tetlet.2012.08.079 |
| [39] |
D. Morimoto, K. Sato, K. Saito, et al., J. Photochem. Photobiol. A 337 (2017) 112-117. DOI:10.1016/j.jphotochem.2017.01.023 |
| [40] |
K. Sato, S. Hagiwara, D. Morimoto, et al., J. Photochem. Photobiol. A 313 (2015) 9-14. DOI:10.1016/j.jphotochem.2015.05.015 |
| [41] |
D. Morimoto, H. Yoshida, K. Sato, et al., Langmuir 33 (2017) 3680-3684. DOI:10.1021/acs.langmuir.7b00512 |
| [42] |
N. Sun, K. Su, Z. Zhou, et al., ACS Appl. Mater. Interfaces 10 (2018) 16105-16112. DOI:10.1021/acsami.8b01624 |
| [43] |
Z. Wang, W. Bai, J. Tong, et al., Chem. Commun. 52 (2016) 10365-10368. DOI:10.1039/C6CC02851A |
| [44] |
Y. Cheng, J. Wang, Z. Qiu, et al., Adv. Mater. 29 (2017)1703900.
|
| [45] |
C. Duan, M. Won, P. Verwilst, et al., Anal. Chem. 91 (2019) 4172-4178. DOI:10.1021/acs.analchem.9b00224 |
| [46] |
J. Wu, L. Jiang, P. Verwilst, et al., Chem. Commun. 55 (2019) 9947-9950. DOI:10.1039/C9CC05048E |
| [47] |
Y. Xu, B. Li, W. Li, et al., Chem. Commun. 49 (2013) 4764-4766. DOI:10.1039/c3cc41994k |
| [48] |
Y. Su, W. Shi, X. Chen, et al., RSC Adv. 6 (2016) 41340-41347. DOI:10.1039/C6RA06942H |
 2019, Vol. 30
2019, Vol. 30 

