Ultralong organic phosphorescence (UOP) materials, which are organic persistent luminescence, have attracted great attention and been applied in the fields of organic light-emitting devices [1, 2], biological imaging [3], optical recording [4], information encryption [5] and sensors [6], etc. Although UOP materials possess great advantages including facile synthetic routines and methods, good solubility for wet chemistry processing, low toxicity, biocompatibility, sustainability metal-free [7-10], the lifetimes are not long enough to compete with the conventional persistent luminescence materials [11, 12]. The difficulties to reach long UOP lifetimes were not only ascribed to the intrinsicly spin-forbidden phosphorescence process and the deactivation of triplet excitons owing to thermodynamic energy dissipation and environemental oxygen quenching [13, 14], but also imputed to the ambiguous relationship and principle among the diversity of molecular configurations, aggregation modes, and phosphorescence lifetimes supporting without prospective guidance. Among the reported strategies used to promote the luminescent properties of organic phosphorescent materials, one is to optimize the UOP efficiency by reinforcing spin orbit coupling [15, 16] with adjustable substitutions of aromatic carbonyls [17, 18], or heavy halogen [17, 19-25]. Another aspect is to prolong UOP lifetimes through suppressing the nonradiative decay process [26] from triplet to ground state, which were generally stabilized by crystallization [27, 28], H-aggregation [5, 29], deuterium, rigid matrix [30-33], polymerization and host-guest methods, etc. [34-38]. Beside of that, many molecular structures including carbazol-9-yl-based amides and triazines [39], aroyl aromatics [21, 24, 39-42], terephthalate aromatic salts [33], aromatic boronic compounds [43, 44], naphthalene-based amines and imides [45, 46], siloles [18], cyclic triimidazoles [29], diarylamines [47], halogen aromatics and their cocrystals [20, 32, 35, 48, 49], sulphurous [50-52] and phosphorus heterocycles [53], etc. have been found to be organic phosphors with long UOP lifetimes. But few of the reports clarified the intrinsic relationship among the crystalline regioisomeric phosphors and the UOP lifetimes [37, 54, 55].
Herein, we chose a series of apparently regioisomeric dicarbazol-9-yl pyrazine derivatives (p-DCzP, m-DCzP, and o-DCzP) with para-, meta-, and ortho-convergent substitutions leading to the gradually variable dihedral angles between two carbazol-9-yl moieties presented in Scheme 1, respectively. The electronwithdrawn pyrazine moiety, which has been explored as a central backbone of various organic luminescent semiconductors in the fields of thermal activation delayed fluorescence (TADF) [56] or metal-organic framework (MOF) [57], could potentially explored to be a new series of UOP molecules. In this work, the regioisomerism effect (RIE) of crystalline p-DCzP, m-DCzP, and o-DCzP with convergent substitutions on different pyrazine moieties was disclosed to be the key role inducing the gradually prolonged UOP lifetimes from 63.14 ms, 127.93 ms to 350.46 ms, respectively. The RIE is by far reasonably illustrated according to the packing modes, single crystal analysis and photophysical properties.
Three pyrazine-based UOPs including p-DCzP [58], m-DCzP, and o-DCzP were synthesized by nucleophilic reaction in one step (Scheme S1 in Supporting information). Chemical structures were fully characterized by 1H NMR (Figs. S1-S3 in Supporting information), mass spectrometry (Figs. S4-S6 in Supporting information), and the single crystal X-ray diffraction data (Table S1 in Supporting information). The possible substitution on pyrazine cores by two carbazol-9-yls make them configurationally different regioisomers. The dihedral angles between two carbazol-9-yl moieties with the p-, m- and o-convergent substitutions were 180°, 131.46° and 63.07° for p-DCzP, m-DCzP, and o-DCzP, respectively (Fig. 1). Their increasing dihedral angles illustrated that the sequentially substitutional regioisomerism has an obvious impact on the packing modes for tuning UOP lifetimes of three DCzP derivatives.

|
Download:
|
| Fig. 1. Regioisomerism effect (RIE) of p-DCzP, m-DCzP, o-DCzP-based UOP materials in crystal state. | |
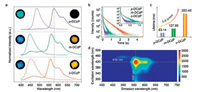
The photophysical properties of three molecules in both solution and solid state were investigated by absorption and photoluminescence (PL) spectra. As shown in Fig. S7a (Supporting information), the absorption spectra in diluted toluene solution were similar for three DCzPs. The absorbance peaks at 330 nm and 370 nm were ascribed to the π–π* transition for the conjugation of carbazol-9-yl groups and n-p* transition for pyrazine ring, respectively. It can be found in Fig. S7a that the emission peaks in solution for p-DCzP, m-DCzP and o-DCzP appeared at 417, 446, and 430 nm. Moreover, as shown in Table S1 and Fig. S7b (Supporting information), the fluorescence lifetimes for p-DCzP, m-DCzP and o-DCzP in solution were measured as 8.05, 7.01 and 9.42 ns, correspondingly. Clearly, these results demonstrated that the RIE has a tiny effect on the optical properties of the three molecules in dilute solution. However, in crystal state, the photophysical characteristics of p-DCzP, m-DCzP and o-DCzP under ambient condition were obviously different. As shown in Fig. 2a, the PL emission bands presented an obvious bathochromic shift in crystals compared with that in solution due to the intermolecular π–π stacking [59]. The inserted images in Fig. 2a showed the color change of the crystals before and after removing the UV lamp. When their crystals were excited under the UV at 365 nm, green-blue, blue, and sky-blue emissions ranging from about 400–600 nm for p-DCzP, m-DCzP and o-DCzP were observed, respectively. After the removal of light source, the emission around 550 nm gradually changed to yellow and lasted for 2 s (Fig. S14 in Supporting information). As shown in Fig. 2a, the phosphorescence spectra of these crystals are identical except for the shorter wavelength emission at the 400–500 nm. These short wavelength emission bands around 490 nm for o-DCzP and 450 nm, 500 nm for m-DCzP are different with the short emission bands of the phosphorescence spectra at 77 K (Fig. S8 in Supporting information), which can be ascribed to the delayed fluorescence due to the triplet-triplet annihilation [39]. As shown in Fig. 2b and Figs. S9- S11 (Supporting information), time-resolved emission spectra revealed a surprising phenomenon that o-DCzP possesses the longest UOP lifetime for 350.46 ms at 562 nm, while p-DCzP and m-DCzP have 63.14 ms at 580 nm, 127.93 ms at 562 nm, respectively. Notably, when the substitution site changes from p- to o-position, the UOP lifetime for o-DCzP was almost 5.5 times longer than that of p-DCzP. Fig. 2d and Fig. S12 (Supporting information) showed excitation-phosphorescence-mapping to further understand the UOP phenomenon in crystal states. When excited from 280 nm to 500 nm, the intensity of short wavelength around 400–500 nm was reduced, but the intensity at around 550 nm was increased, which was assigned to the UOP. Furthermore, on the basis of single crystal X-ray diffraction analysis, the angles of transition dipoles and the interconnected axis (θ) were larger than the critical value of 54.7° that was used to distinguish H- from J-aggregates [5, 60]. The dipole moment angles calculated from single crystals were 75.94° for o-DCzP, 83.28° for p-DCzP and 60.49° for m-DCzP which were all larger than 54.7° (Fig. S13 in Supporting information). Hence, molecules in the crystal states which formed H-aggregated could stabilize the triplet excitons to realize UOP under ambient condition [23-25, 46].
|
Download:
|
| Fig. 2. Photophysical properties of crystalline p-DCzP, m-DCzP, and o-DCzP under ambient condition. (a) The photoluminescence spectra (dashed lines) and phosphorescence (solid lines) excited at 365 nm. Insets showed the photographs taken before (left) and after (right) the excitation light source was turned off, respectively. (b) Phosphorescence decay curves of p-DCzP, m-DCzP, and o-DCzP at 562 nm (λEX = 365 nm), 580 nm (λEX = 365 nm), and 560 nm (λEX = 380 nm), respectively. (c) The increasing tendency of phosphorescence lifetimes of p-DCzP, m-DCzP, and o-DCzP. (d) Excitation-phosphorescence-mapping of o-DCzP in crystal state. The color change from red to green indicated the decrease in the emission intensity. | |
To find out the origin of the ultralong phosphorescence of three crystals in this study, the intra-/intermolecular interactions in the single crystals were investigated. As expected, the intra/intermolecular interactions of p-DCzP, m-DCzP and o-DCzP are different because of the RIE. Nonradiative transitions and exciton quenching through molecular interactions with the surrounding environment (humidity, oxygen, etc.) can be effectively prevented by crystallization [61]. Figs. 3a-c presents the single crystal structures, there were three different strong intramolecular C— H ⋯ N interactions with distances ranging from 2.380 Å to 2.693 Å around pyrazine cores for p-DCzP, m-DCzP and o-DCzP in single molecule, which contributed to suppress molecular rotation to reduce the nonradiative decay of the excitons. As shown in Figs. 3d-f, the main intermolecular interactions between two adjacent dimers were consisted of multiple p ⋯π, C— H ⋯π, C— H ⋯ H— C, and C— H ⋯ N interactions. In crystal of o-DCzP, molecules adopt twisted conformations as equilateral triangle with steric repulsion. The intermolecular interactions are symmetrically distributed on both of the carbazol-9-yl groups between the neighbour molecules. The strong C— H ⋯π interaction (2.860 Å ) exists between the carbazole ring of two adjacent molecules (Fig. 3f). Moreover, kinds of π ⋯π interactions with distances of 3.315(2), 3.278(4) and 3.341(2) Å were observed between the carbazole in o-DCzP and the neighbour molecules. Hence, the rotational freedom of two carbazol-9-yl group was firmly restricted by those intermolecular interactions. For p-DCzP, symmetric substitution of carbazole results in a nearly planar configuration of the entired molecule. The intermolecular interactions of p-DCzP were mainly focused on the central pyrazine ring and on the carbazol-9-yl group near the central ring through a vertical molecular arrangement via C— H ⋯ H— C interactions (2.352(4) Å ) and horizontal molecular packing via C— H ⋯π interactions with distances of 2.674(3), 2.851 (3) Å and C— H ⋯ H— C interactions (2.034(3) Å ) between the two adjacent molecules, respectively. In m-DCzP, C— H ⋯π intermolecular interactions (2.866(2), 2.878(2) and 2.895(2) Å ) along the vertical orientation between pyrazine cores and the carbazol-9-yl group of the adjacent dimers were obtained. Another C— H ⋯π intermolecular between the carbazol-9-yl group in adjacent molecules was 2.317(2) Å . To be specific, another C— H ⋯ N (2.634 Å ) interaction is observed between pyrazine cores and the adjacent dimers. From the molecular packing modes, the intermolecular interactions of p-DCzP and m-DCzP molecules were dispersed in the central pyrazine ring and the side carbazol-9-yl group, resulting in that their molecular rotations are partially fixed in the crystalline state. It is worthy to note that the RIE could lead to three types strong p ⋯π interactions (3.278, 3.315 and 3.341 Å ) in the horizontal position for o-DCzP and a strong C— H ⋯ N (2.634 Å ) interaction for m-DCzP. The strong intra-/intermolecular interactions in o-DCzP based on near equilateral triangule configuration were beneficial to rigid molecular conformations [46].

|
Download:
|
| Fig. 3. (a–c) Crystalline molecular configurations and (d–f) crystalline molecular stacking of p-DCzP, m-DCzP and o-DCzP in the crystal state, respectively. Note that the dashed lines represented the intramolelar and intermolecular interactions. | |
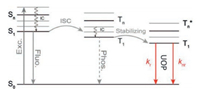
The above results revealed that the longest phosphorescence lifetime τp = 1/(kr+knr) in o-DCzP crystal was likely stemmed from the optimized radiative and nonradiative decay [29, 42] (Fig. 4). In a word, RIE successfully tuned molecular electronic states and corresponding molecular environment with tunable UOP lifetimes in crystal state.
In order to take full advantage of three UOP molecules, we applied them via an anti-counterfeiting technology [39, 50, 51]. Persistent emission from UOP materials could enable the usage of different temporal information. The emission gradually decreased after variable excitation with different times from where the longlived emitting materials were casted. The temporal information loaded with UOP materials increased the difficulty to distinguish and decode the ultralong phosphorescent emission for potential low-cost photodetection and information coding systems [62]. Three molecules with different phosphorescence lifetimes and emission could be seen with the naked eyes after switching off the UV lamp. UOP materials could also be used as security information carriers. As shown in Fig. 5b, aqueous slurry was prepared by stirring with o-DCzP powder, coated on a stencil and printed on a paper. When the UV lamp was switched off, the active layer retained significant afterglow characteristics. As a result, UOP materials enable "pattern-to-pattern" batch processing with the simple stencil printing.
In summary, this work presented the RIE existing in a series of crystalline dicarbazol-9-yl-based pyrazines with regular phosphorescence lifetimes. The RIE led to the longest lifetime (350.46 ms) of crystalline o-DCzP with the closest dicarbazoly-9-yls which was about 5.5 times longer than that of p-DCzP due to the strongest intra-/intermolecular interactions. Theoretical calculation on the basis of multimolecular systems will be carried out to deeply investigate and get closer to the primary source of RIE. It is believed that the RIE could provide a new perspective to insight into the mechanism of UOP properties and explore more advanced UOP mateirals [63-67].
|
Download:
|
| Fig. 4. Jablonski diagram of the relevant photophysical processes in UOP system. IC: Inner crossing; ISC: Intersystematic crossing; T1-Tn: triplet excitons at different energy level; S0-Sn: singlet excitons at different energy level; Exc.: Excitation; Fluo.: Fluorescence; Phos.: Phosphorescence. | |

|
Download:
|
| Fig. 5. (a) "NJTECH" was spelled using the o-DCzP, m-DCzP, and p-DCzP single crystals. The typical duration of afterglow was explored as time-resolved anti-counterfeiting. (b) "Chinese knotting" pattern was printed on paper and last for seconds when the UV lamp was turned off in a darkroom. | |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 21975126, 51673095, 21875104, 21875191, 21603104), the Natural Science Foundation of Jiangsu Province (Nos. BK20171470, BK20160991, BK20150064, BK20130912), 973 Program (No. 2015CB932200) and Ministry of Education and Synergetic Innovation Center for Organic Electronics and Information Displays for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.09.005.
| [1] |
G. He, W.T. Delgado, D.J. Schatz, et al., Angew. Chem. Int. Ed. 53 (2014) 4587-4591. DOI:10.1002/anie.201307373 |
| [2] |
S. Reineke, M.A. Baldo, Sci. Rep. 4 (2014) 3797.
|
| [3] |
J. Samonina-Kosicka, C.A. DeRosa, W.A. Morris, et al., Macromolecules 47 (2014) 3736-3746. DOI:10.1021/ma5006606 |
| [4] |
A. Kishimura, T. Yamashita, K. Yamaguchi, T. Aida, Nat. Mater. 4 (2005) 546-549. DOI:10.1038/nmat1401 |
| [5] |
Z. An, C. Zheng, Y. Tao, et al., Nat. Mater. 14 (2015) 685-690. DOI:10.1038/nmat4259 |
| [6] |
Z. Chen, K.Y. Zhang, X. Tong, et al., Adv. Funct. Mater. 26 (2016) 4386-4396. DOI:10.1002/adfm.201600706 |
| [7] |
Q. Li, Z. Li, Adv. Sci. 4 (2017) 1600484.
|
| [8] |
Z. Li, Sci. China Chem. 58 (2015) 969.
|
| [9] |
S. Xu, R. Chen, C. Zheng, W. Huang, Adv. Mater. 28 (2016) 9920-9940. DOI:10.1002/adma.201602604 |
| [10] |
W. Zhao, Z. He, J.W.Y. Lam, et al., Chem 1 (2016) 592-602. DOI:10.1016/j.chempr.2016.08.010 |
| [11] |
S. Lamansky, P. Djurovich, D. Murphy, et al., J. Am. Chem. Soc. 123 (2001) 4304-4312. DOI:10.1021/ja003693s |
| [12] |
Y.H. Niu, Y.L. Tung, Y. Chi, et al., Chem. Mater. 17 (2005) 3532-3536. DOI:10.1021/cm047709f |
| [13] |
Z. Xia, A. Meijerink, Chem. Soc. Rev. 46 (2017) 275-299. DOI:10.1039/C6CS00551A |
| [14] |
H. Xu, R. Chen, Q. Sun, et al., Chem. Soc. Rev. 43 (2014) 3259-3302. DOI:10.1039/C3CS60449G |
| [15] |
M. Porel, M.F. Ottaviani, S. Jockusch, et al., Chem. Commun. 46 (2010) 7736-7738. DOI:10.1039/c0cc02587a |
| [16] |
C.M. Marian, Wires Comput. Mole. Sci. 2 (2012) 187-203. DOI:10.1002/wcms.83 |
| [17] |
S. Hirata, K. Totani, J. Zhang, et al., Adv. Funct. Mater. 23 (2013) 3386-3397. DOI:10.1002/adfm.201203706 |
| [18] |
W.Z. Yuan, X.Y. Shen, H. Zhao, et al., J. Phys. Chem. C 114 (2010) 6090.
|
| [19] |
Z. Mao, Z. Yang, Y. Mu, et al., Angew. Chem. Int. Ed. 54 (2015) 6270-6273. DOI:10.1002/anie.201500426 |
| [20] |
H. Shi, Z. An, P.Z. Li, et al., Cryst. Growth Des. 16 (2016) 808-813. DOI:10.1021/acs.cgd.5b01400 |
| [21] |
O. Bolton, D. Lee, J. Jung, J. Kim, Chem. Mater. 26 (2014) 6644-6649. DOI:10.1021/cm503678r |
| [22] |
O. Bolton, K. Lee, H.J. Kim, K.Y. Lin, J. Kim, Nat. Chem. 3 (2011) 205-210. DOI:10.1038/nchem.984 |
| [23] |
M.S. Kwon, D. Lee, S. Seo, J. Jung, J. Kim, Angew. Chem. Int. Ed. 53 (2014) 11177-11181. DOI:10.1002/anie.201404490 |
| [24] |
D. Lee, O. Bolton, B.C. Kim, et al., J. Am. Chem. Soc. 135 (2013) 6325-6329. DOI:10.1021/ja401769g |
| [25] |
D. Lee, X. Ma, J. Jung, et al., Phys. Chem. Chem. Phys. 17 (2015) 19096-19103. DOI:10.1039/C5CP01003A |
| [26] |
H.A. Al-Attar, A.P. Monkman, Adv. Funct. Mater. 22 (2012) 3824-3832. DOI:10.1002/adfm.201200814 |
| [27] |
X. Pang, H. Wang, X.R. Zhao, W.J. Jin, CrystEngComm 15 (2013) 2722-2730. DOI:10.1039/c3ce26661c |
| [28] |
M. Shimizu, A. Kimura, H. Sakaguchi, Eur. J. Org. Chem. 2016 (2016) 467-473. DOI:10.1002/ejoc.201501382 |
| [29] |
E. Lucenti, A. Forni, C. Botta, et al., J. Phys. Chem. Lett. 8 (2017) 1894-1898. DOI:10.1021/acs.jpclett.7b00503 |
| [30] |
X. Chen, C. Xu, T. Wang, et al., Angew. Chem. Int. Ed. 55 (2016) 9872-9876. DOI:10.1002/anie.201601252 |
| [31] |
M. Koch, K. Perumal, O. Blacque, et al., Angew. Chem. Int. Ed. 53 (2014) 6378-6382. DOI:10.1002/anie.201402199 |
| [32] |
J. Wei, B. Liang, R. Duan, et al., Angew. Chem. Int. Ed. 55 (2016) 15589-15593. DOI:10.1002/anie.201607653 |
| [33] |
Z. Cheng, H. Shi, H. Ma, et al., Angew. Chem. Int. Ed. 57 (2018) 678-682. DOI:10.1002/anie.201710017 |
| [34] |
J. Cao, X. Ma, M. Min, et al., Chem. Commun. 50 (2014) 3224-3226. DOI:10.1039/C3CC49820D |
| [35] |
D. Li, F. Lu, J. Wang, et al., J. Am. Chem. Soc. 140 (2018) 1916-1923. DOI:10.1021/jacs.7b12800 |
| [36] |
J. Kuijt, F. Ariese, U.A.T. Brinkman, C. Gooijer, Anal. Chim. Acta 488 (2003) 135-171. DOI:10.1016/S0003-2670(03)00675-5 |
| [37] |
W. Luo, Y. Zhang, Y. Gong, et al., Chin. Chem. Lett. 29 (2018) 1533-1536. DOI:10.1016/j.cclet.2018.08.001 |
| [38] |
Y. Xie, Y. Ge, Q. Peng, et al., Adv. Mater. 29 (2017) 1606829.
|
| [39] |
L. Gu, H.F. Shi, L.F. Bian, et al., Nat. Photonics 13 (2019) 406-411. DOI:10.1038/s41566-019-0408-4 |
| [40] |
M.S. Kwon, Y. Yu, C. Coburn, et al., Nat. Commun. 6 (2015) 8947.
|
| [41] |
Y. Yu, M.S. Kwon, J. Jung, et al., Angew. Chem. Int. Ed. 129 (2017) 16425-16429. DOI:10.1002/ange.201708606 |
| [42] |
N. Gan, X. Wang, H. Ma, et al., Angew. Chem. Int. Ed. (2019) 14140-14145.
|
| [43] |
Z. Chai, C. Wang, J. Wang, et al., Chem. Sci. 8 (2017) 8336-8344. DOI:10.1039/C7SC04098A |
| [44] |
Y. Shoji, Y. Ikabata, Q. Wang, et al., J. Am. Chem. Soc. 139 (2017) 2728-2733. DOI:10.1021/jacs.6b11984 |
| [45] |
S. Hirata, M. Vacha, J. Phys. Chem. Lett. 7 (2016) 1539-1545. DOI:10.1021/acs.jpclett.6b00554 |
| [46] |
M. Shimizu, T. Kinoshita, R. Shigitani, Y. Miyake, K. Tajima, Mater. Chem. Front. 2 (2018) 347-354. DOI:10.1039/C7QM00524E |
| [47] |
H. Shi, X. Ma, Q. Zhao, et al., Adv. Funct. Mater. 24 (2014) 4823-4830. DOI:10.1002/adfm.201400647 |
| [48] |
C.R. Wang, Y.Y. Gong, W.Z. Yuan, Y.M. Zhang, Chin. Chem. Lett. 27 (2016) 1184-1192. DOI:10.1016/j.cclet.2016.05.026 |
| [49] |
L. Xiao, Y. Wu, J. Chen, et al., J. Phys. Chem. A 121 (2017) 8652-8658. |
| [50] |
Z. Yang, Z. Mao, X. Zhang, et al., Angew. Chem. Int. Ed. 55 (2016) 2181-2185. DOI:10.1002/anie.201509224 |
| [51] |
Z. He, W. Zhao, J.W.Y. Lam, et al., Nat. Commun. 8 (2017) 416.
|
| [52] |
B. Xu, H. Wu, J. Chen, et al., Chem. Sci. 8 (2017) 1909-1914. DOI:10.1039/C6SC03038F |
| [53] |
P. Xue, P. Wang, P. Chen, J. Ding, R. Lu, RSC Adv. 6 (2016) 51683-51686. DOI:10.1039/C6RA07477D |
| [54] |
K. Schmidt, S. Brovelli, V. Coropceanu, et al., J. Phys. Chem. A 111 (2007) 10490-10499. DOI:10.1021/jp075248q |
| [55] |
Q. Xiong, C. Xu, N. Jiao, et al., Chin. Chem. Lett. 30 (2019) 1387-1389. DOI:10.1016/j.cclet.2019.04.010 |
| [56] |
K. Kawasumi, T. Wu, T. Zhu, et al., J. Am. Chem. Soc. 137 (2015) 11908-11911. DOI:10.1021/jacs.5b07932 |
| [57] |
S. Khatua, A.K. Bar, J.A. Sheikh, A. Clearfield, S. Aonar, Chem. -Eur. J. 24 (2018) 872-880. DOI:10.1002/chem.201704088 |
| [58] |
J.Q. Jin, G.K. Long, Y.Q. Gao, et al., ACS App. Mater. Interfaces 11 (2019) 1109-1116. DOI:10.1021/acsami.8b16561 |
| [59] |
T.M. Figueira-Duarte, K. Müllen, Chem. Rev. 111 (2011) 7260-7314. DOI:10.1021/cr100428a |
| [60] |
S. Cai, H. Shi, J. Li, et al., Adv. Mater. 29 (2017) 1701244.
|
| [61] |
M. Shimizu, R. Shigitani, M. Nakatani, et al., J. Phys. Chem. C 120 (2016) 11631-11639. DOI:10.1021/acs.jpcc.6b03276 |
| [62] |
Y. Deng, D. Zhao, X. Chen, et al., Chem. Commun. 49 (2013) 5751-5753. DOI:10.1039/c3cc42600a |
| [63] |
Z. He, W. Li, G. Chen, et al., Chin. Chem. Lett. 30 (2019) 933-936. DOI:10.1016/j.cclet.2019.03.015 |
| [64] |
S. Hirata, Adv. Opt. Mater. 5 (2017) 1700116.
|
| [65] |
C. Wang, Y. Gong, W. Yuan, et al., Chin. Chem. Lett. 27 (2016) 1184-1192. DOI:10.1016/j.cclet.2016.05.026 |
| [66] |
H. Ma, W. Shi, J. Ren, et al., J. Phys. Chem. Lett. 7 (2016) 2893-2898. DOI:10.1021/acs.jpclett.6b01156 |
| [67] |
P. Zhao, L. Zhu, Chin. Chem. Lett. 29 (2018) 1706-1708. DOI:10.1016/j.cclet.2018.10.034 |
 2019, Vol. 30
2019, Vol. 30 

