b Key Laboratory for Organic Electronics and Information Displays & Institute of Advanced Materials(IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials(SICAM), Nanjing University of Posts & Telecommunications, Nanjing 210023, China;
c Shaanxi Institute of Flexible Electronics(SIFE), Northwestern Polytechnical University(NPU), Xi'an 710072, China;
d State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, China;
e Madrid Institute for Advanced Studies(IMDEA Nanociencia), Ciudad Universitaria de Cantoblanco, Calle Faraday 9, Madrid 28049, Spain
Conjugated polymers (CPs) have attracted more attentions in the past decades for their potential applications in solution-processed plastic optoelectronic devices, included polymer light-emitting diodes (PLEDs), polymer laser, polymer solar cell (PSC) and polymer memory [1-8]. Many effective molecular design strategies have been proposed to construct high performance and stable CPs via precisely controlling electronic structures [6, 8-11]. Beyond the tailorable molecular p-n engineering, aggregation behavior of CPs chain also play a key role to control photophysical processing, exciton diffusion and charge transport [4, 5, 12-15]. Compared to the deposited films of small molecules, film morphology of CPs can be tuned by solution formulations, fabrication technology and post-processing [3, 7, 12, 16, 17]. The precursory aggregation structure can be developed in the solution that determines the structural rearrangement in the film-casting process [4, 16, 18-20]. Up to date, various types of side chains have been introduced into CPs to improve solubility and tune film morphology, such as alkyl chains, hybrid, oligoether, fluoroalkyl, and latently reactive side chains [21-26]. Traditionally, side chains are used to regulate the solubility of the backbone of CPs. For example, increasing the length of alkyl chain for CPs apparently enhanced their solubility in organic solvent, which brings plastic optoelectronics the advantages of low cost, large area, and flexibility [10, 27]. Besides, the aggregation behavior of the CPs is dramatically influenced by the solubility, which intrinsically resulted from the property of side chains [21, 28]. Therefore, side chains generate the variation of chain aggregation behavior, which is the main factor affecting the behavior of interchain packing, crystalline, film morphology, and photoelectrical properties.
As an important light-emitting conjugated polymer, polyfluorene has been widely applied in PLEDs and laser for their easily modification, deep-blue emission and high luminance efficiency [5, 10, 16, 29, 30]. Structural functionalizations at 9-, 4-sited position of fluorene enable chain to show excellent polymorphism behavior and variable phase in condensed structure, to improve materials optoelectronic property and further meet the various require-ments of different devices [16, 21, 31-34]. The balance of interchain aggregation and steric hindrance in polyfluorene is the preliminary factor to induce abundant chain conformation and aggregation behavior in solution states [35]. Among all structural element, side chain is crucial factor to control interchain aggregation and tune chain conformation in solution and film states [21, 33, 36]. Systematical exploration in polydialkylfluorenes (PFn) and bulky polydiarylfluorenes (PnDPF, n = 6, 7, 8, 9) all-inclusive, indicated that the octyl chain was the most advantageous length to cause strong order aggregation, obtain planar conformation and crystalline structure in the film state [16, 21, 32, 33, 36-41]. Compared to the linear type side chain, the branched one always gives rise to excellent solution ability for their flexible ability, which can also precisely control molecular arrangement and photophysical processing in condensed structures [27, 42]. In this work, we designed and prepared two polydiarylfluorenes to explore the alkyl-chain branched effect on the aggregation and photophysical behavior toward stable and efficient deep-blue electroluminescence (EL) and amplified spontaneous emission (ASE). Optical analysis associate with dynamic light scattering (DLS) were applied to study aggregation and photophysical behavior in solution states. Finally, efficient and stable deep-blue EL and ASE behaviour also been explored to confirm the branched effect.
To demonstrate the alkyl-chain branched effect on the aggregation and photophysical behavior, we introduce the different branched alkyl chains with same carbon atom at 4-position of fluorene monomer (Fig. 1). Based on our previous works [7, 30, 40, 43], all polymers were prepared by Yamamoto polymerization (Fig. 1a). Details of the polymer synthesis are shown in the Supporting Information (Fig. S1 in Supporting information). The 1H NMR spectra of three polymers are displayed in Fig. S2 (Supporting information) to confirm their chemical structures. These polymers appeared the similar chemical shifts in the aromatic region, while in the high field region they have the different integral peaks due to the different of branched side chain. For the PEODPF, carbon adjacent to the oxygen atom at 4-possition, has two hydrogen atoms on it at about 4.0 ppm, and the POYDPF sample's carbon adjacent to the oxygen has one hydrogen atom. Their molecular weights, polydispersity indices (PDI) and thermodynamic data are listed in Table 1. The number average molecular weight (Mn) and index of polydispersity (PDI) of the three polymers were determined by gel permeation chromatography (GPC) to be about 21-39 kDa and 1.95-2.3, respectively. These three polymers had similar molecular weight and PDI demonstrated that molecular weight had no effect on their various properties of polyfluorenes. The glass transition temperature (Tg) is an indispensable parameter of polymer. The Tg of these polymers were ~171 ℃ for PEODPF, ~178 ℃ for POYDPF, ~200 ℃ for PODPF (Fig. S3 in Supporting information), respectively, according to the DSC measurement, which is the effective method for recording Tg, respectively. Notably, the PEODPF exhibited a lower Tg of 171 ℃ than those of PODPF, suggested the flexible property induced by branched effect. From Table 1 we can see, the Tg of PEODPF and POYDPF were lower than PODPF. In this regard, compared to branched polymer, liner-type PODPF had excellent phase and conformational transitions up thermal annealing treatment [32].

|
Download:
|
| Fig. 1. (a) Chemical structures of three polymers. UV-vis absorption (b) and PL (c) spectra of three polymers in diluted toluene solutions (10-3 mg/mL). | |
|
|
Table 1 Summary of the structural data of the three polymers. |
In order to investigate the chain behavior and photophysical properties of the three polymers, their UV-vis absorption and photoluminescence (PL) spectra in diluted toluene solution are illustrated in Fig. 1. As displayed in Fig. 1b, the absorption spectra of the three polymers in diluted solution have similar absorption peaks at about 392 nm, attributed to their π→π* transitions of polyflourenes mainchain [20, 33, 36]. According to our previous works [30, 32, 38, 40, 41], the shoulder absorption at about 440 nm was observed in PODPF diluted toluene solution with a Mn of 65.7 kDa, associated with the characteristic β-conformation absorption peak. However, this shoulder absorption peak cannot observe in Fig. 1b, revealed the no β-conformation formation for our type PODPF in this work. In fact, as we studied, the formation ability of β-conformation in PODPF presents significantly was enhanced with increasing molecular weight [32, 33, 40]. As presented in Fig. 1c, the PL spectra of the three polymers have similar emission peaks at about 429 nm, 454 nm, and 493 nm with similar life decay time (Fig. S4 and Table S1 in Supporting information), assigned to 0-0, 0-1, 0-2 π*-π transitions of the typical single polyfluorene chains [20, 33], all of the polymer chains are well-dissolved in the isotropic phase. Therefore, it is easy to conclude the slight branched effect on photophysic behavior of single polyfluorenes chain in diluted solutions.
According to previous works [7, 12, 16, 19, 20, 30, 35, 40], aggregation will induce the formation of interchain excited state in condensed structure and dominated their optical property. Therefore, combining the optical analysis, dynamic light scattering (DLS) are introduced here to investigate the aggregation-dependent photophysical processing in solution sates. As displayed in Fig. 2, with increasing the concentration from 10-3 mg/mL to 0.5 mg/mL, similar PL spectra are obtained for the PEODPF, and POYDPF with slightly strong 0-1 band emission, compared to the diluted solution, associated with the self-absorption or weak aggregation [7, 16, 35, 44]. However, charac-teristic β-conformational PL spectra are found in the PODPF concentrated toluene solution, similar to the previous works (Fig. 2b) [7, 16, 20, 35]. Besides, we also explored the corresponding interchain aggregation behavior in this concentrated solution by DLS measurements (Fig. 2a) [33, 35, 36, 45]. The correlation function (g2 (t)-1) and the hydrodynamic radius distribution (Rh) of the PEODPF, POYDPF and PODPF in concentrated toluene solutions at 25 ℃ are displayed in Fig. 2. In accordance with DLS data, two types of relaxation time are appeared, one is POYDPF, and the other is PEODPF and PODPF. First of all, the correlation function of PEODPF had a short relaxation time with a Rh of estimated about ~10 nm, consistent with the extended and persistence length of individual chain [33, 35], consistent with the optical analysis. Meanwhile, this extended and rigid chain state also support the excellent solubility of PEODPF in this concentrated toluene solution. However, although the similar PL spectra of POYDPF and PEODPF are observed in solution, additional longer relaxation model at Rh = ~ 100 nm, is obtained for the POYDPF type solution, indicated the formation of nano-aggregate in the solution states [16, 33, 35]. But this weak aggregation is inefficient enough to induce the formation of interchain excited state, reasonable explain the similar PL spectra of POYDPF and PEODPF. Therefore, we design a chain behavior model of three polymers in the toluene solution to further describe the aggregation behavior (Fig. 2c). From the model, we can see the PEODPF shown a single chain behavior, but the PODPF demon-strated strongest interchain aggregation behavior, and the POYDPF both simultaneously. From the above results, we infer that polymer chain of POYDPF into aggregates may due to the lower solubility than PEODPF. The situations for PODPF are significantly different that there is only longer relaxation model with an Rh value of ~100 nm, indicating stronger interchain aggregation than those of POYDPF and PEODPF [35]. These interchain aggregations provided a platform to stabilize the formation of β-conformation in concentrated solution in accordance with the optical analysis [21]. Therefore, PODPF exhibited strong interchain aggregation than those of POYDPF and PEODPF in solution, which is a key factor to dominate the film morphology and photophysical property.

|
Download:
|
| Fig. 2. (a) DLS curves of three polymers in toluene solutions with a concentration of 0.5 mg/mL, (b) together with their PL spectra and aggregation model in solution states. (c) Chain behaviour model of three polymers in the toluene solution. | |
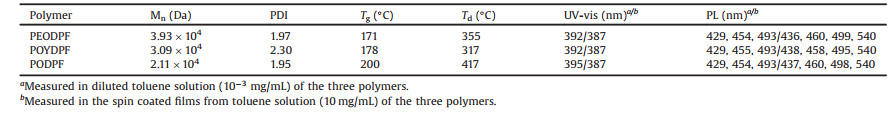
Beyond the solutions state, we also investigated the photo-physical property of three polymers in spin-coated and annealed film. As displayed in Fig. 3, maximum absorption peak of three polymer pristine films are estimated about 390 nm (Fig. 3a), associated with the polyfluorene mainchain [20, 33]. And corresponding PL spectra also consisted of four emission peaks at 440, 467, 500 and 540 nm, confirmed the amorphous states in the pristine films [20, 32]. However, compared to the POYDPF ones, the well-resolved emission peaks for PEODPF and PODPF also indicated the weaker interchain aggregation or relatively oriented chain-chain arrangement. Meanwhile, upon decreasing the analysis temperature, as result of the freezing out of torsional modes, these well-resolved emission peaks in low-temperature PL spectra are observed for all pristine spin-coated films (Fig. 3b) [13, 21], also confirmed this assumption. Obviously, there is no planar conformation obtained in all three polymer pristine films. It is a common sense that there is no enough time to allow for adjust chain rearrangement in the spin-coated processing for the fast volatilization of solvent molecules, which can reasonably explain the amorphous state for the PODPF pristine spin-coated films [32, 33, 40]. As we discussed above, three polymer chains may rearrange and adjust each other packing model in condensed structures upon thermal annealing [32]. Therefore, we also explored the branched effect on aggregation and photophysical property via thermal annealing pristine films at 220 ℃ in the air atmosphere. It is easily to find that the maximum absorption peak of PEODPF and POYDPF annealed films is about ~395 nm, similar to the pristine films (Fig. 3c). However, we can also see clearly absorbance peak at 411 nm and 440 nm for the annealed films of PODPF, but absent in PEODPF and POYDPF. As presented in Fig. 3d, the PL spectra of the PEODPF and POYDPF polymers have similar emission peaks at about 440, 459, and 499 nm, which were assigned to 0-0, 0–1, 0–2 π*-π transitions of the typical amorphous polyfluorene chains [20]. However, the red-shift PL spectra of PODPF consisted of three peaks at 455, 482, 515 nm, attributed to the planar β-conformation [32, 40]. Therefore, branched PEODPF and POYDPF appeared a higher luminance efficiency about 36.1% and 39.5%, enhanced about 20% than those of PODPF (22.6%) but similar decay time and radiate rate (Fig. S4 and Table S1 in Supporting information). Besides, Raman spectroscopy is also introduced to probe these assumption (Fig. S5 in Supporting information) [32]. The enhancement of the intensity of peaks form from 1290 cm -1 to 1305 cm -1 occurred in PODPF annealed films, also confirmed the formation of β-conformation. Nevertheless, no obvious shift are observed in Raman spectra of PEODPF and POYDPF annealed films, also indicated no obvious conformational transition under thermal annealing. Therefore, it is effectively concluded that corporation of branched side chain will suppress the β-conformation forming ability of polydiarylfluorenes. On the other sides, these branched chains are also inhibited interchain aggregation to improve morphological and spectral stability.
|
Download:
|
| Fig. 3. Optical properties of three polymers in various states. (a) UV-vis absorption and photoluminescence (PL) spectra of three polymer pristine spin-coated film. (b) PL spectra of three polymer pristine film at low temperature (77 K). UV-vis absorption (c) and photoluminescence (PL) (d) spectra of three polymer annealed film. | |
According to DLS and optical analysis of three polymers above (Fig. 2), PEODPF, POYDPF and PODPF chain presented various different aggregation behaviors in the solution and film states. Therefore, we further explored the detailed morphology of their pristine film and annealed films by AFM and XRD measurements. From the Fig. 4, all pristine films had smooth surface, with a roughness of 0.462 nm for PEODPF, 0.669 nm for POYDPF and 0.480 nm for PODPF, respectively. However, after thermal annealing, three annealed films had different film surface. As we expected, similar to pristine films, PEODPF annealed film also present smooth surface with a slightly higher roughness of 0.819 nm, consistent with the optical and DLS analysis, also indicated the introduction of isooctyl chain can improve film forming ability and morphological thermal stability. According to DLS analysis of POYDPF and PODPF, nano-aggregate (Rh = ~100 nm) is formed in the precursor solution, which may maintain in the solution-processed film and dominate their morphology [12, 19, 33, 36, 38, 40]. This rough morphology will be enhanced under thermal treatment at high temperature. Therefore, several nano- and micro-domain are observed in the both annealed film of POYDPF and PODPF. Corresponding roughness are estimated about 1.116 nm and 2.523 nm respectively. As reported in our previous works [19, 32, 33], the crystalline structure was induced in the film after thermal annealing. Therefore, PEODPF exhibited much less aggregates compared to that of POYDPF and PODPF. Besides, X-ray diffraction is used as a research method to obtain the information of material morphology. From the XRD spectrum (Fig. S6 in Supporting information), it can be seen that the three polymers power show multiple peaks. And by the curve analysis, the θ angles were determined to be 6.1° and 10.1°, respectively, which the plane spacing of the lattice of PEODPF were calculated to be 7.3 Å and 4.4 Å, according to the Bragg's Law method. According to this method, we can get π-π stacking distance of the three polymers in the Table S2 (Supporting information). The distance of π-π interaction about 7.4 Å, are associated with the alkoxyethyl arrangement. As displayed in Fig. S5, it was also found that the peak width of the PEODPF, POYDPF compared with PODPF, attributed to the weaker interchain interaction. Because of the presence of β-conformation in PODPF, there is special peak at d =9.6 Å, attributed to the interphase arrangement between eight of carbon alkoxane chain and diarylflourene, and the distance between the main polyfluorene chain formed. Therefore, it is further confirmed multiphase formation in PODPF, which is the result of the synergistic effect of diarylfluorene and octyl, and the corresponding frequency multiplication value is 3.8 Å. Therefore, the micro-structure of the prepared samples was characterized by XRD, consistent with the AFM and optical results.

|
Download:
|
| Fig. 4. AFM images of three polymer pristine and annealed films: (a, b) PEODPF pristine and annealed film, (c, d) POYDPF pristine and annealed film and (e, f) PODPF pristine and annealed films, respectively. | |
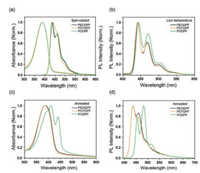
In the last decades, polyfluorene had excellent luminance efficiency and robust deep blue emission, which are widely applications in deep-blue PLEDs and organic lasers [10, 17, 29, 32]. Meanwhile, compared to the solution states, the emission induced by the interchain excited state are significantly amplified in the film state under electrical field, which provided an effective platform to confirm the aggregation behaviour in condensed structures [29, 44]. Therefore, in order to further check this aggregation and morphological dependent EL spectra, we also fabricated a series of PLEDs based on these three emissive polymer pristine and annealed films. The device configuration is shown in Fig. 5a, similar to the previous works. As we expected, our three polymers exhibited excellent EL spectral stability with increasing current density from 0.5 mA/cm2 to 200 mA/cm2. PLEDs based on the PEODPF pristine films had a deep-blue color emission (Fig. 5). And its EL spectra consisted of 436, 459 and 493 nm with a CIE of (0.10, 0.12). Interestingly, corresponding CIE are no obviously change with increasing current density, confirmed the excellent EL spectral stability. Similarly, POYDPF-based PLEDs also present deep-blue emission with a CIE of (0.09, 0.12). Besides, it exhibited similar EL spectra to PEODPF-based PLEDs, included three emission peaks at 435, 459 and 488 nm. However, compared to the PEODPF and POYDPF-based PLEDs, the 0-1 band emission at 457 nm was enhanced for PODPF ones, compared to the 0-1 emission at 440 nm, suggested the strong interchain aggregation in the pristine film and further induce the formation of interchain electron coupling and excited states [7], which is consistent to the results of DLS and XRD analysis. Meanwhile, we also constructed three PLEDs based on their annealed films. Interestingly, PEODPF annealed film present similar EL spectra POYDPF one but weaker 0-1 emission, also confirmed the weaker interchain aggregation in the film states and further allow for single-chain excitonic behavior. However, PODPF annealed films displayed excellent stable feature EL spectra of β-conformation, consistent to the previous work. Besides, compared to the pristine films, PLEDs based on their annealed films present better device performance with a highest current efficiency of 1.57 cd/A at 20 mA/cm2 for PEODPF, 0.97 cd/A at 34 mA/cm2 for POYDPF, and 1.69 cd/A at 12 mA/cm2 for PODPF. It also confirmed the high device performance of β-conformational film than those of amorphous state in polyfluorene. In this regard, branched side-chain can effective inhibit interchain aggregation, which is useful to improve the spectral/morphological stability of efficient deep-blue PLEDs.
|
Download:
|
| Fig. 5. EL spectral stability and device performance of three polymers. (a) Device configuration of the PLEDs. (b) EL spectra of devices based on three polymer pristine (Top) and annealed (Bottom) films, together with their (c) performance of device based on annealed films. | |
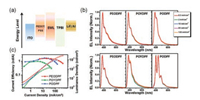
Compared to the amplified energy transfer from host single chain to interchain excited state in PLEDs, ASE behavior are mostly associated with the stable and robust emission efficiency, which is a smart probe to explore the excitonic behavior, phase/conformational transition and morphological stability [10, 17]. Besides, functionalized polydiarylfluorenes is also a potential laser gain medium, attributed to its advantages of high fluorescence efficiency and excellent spectral stability. Therefore, we investi-gated the optical gain performance of the film of the three polymers pristine films upon the thermal annealing. Fig. 6 presented the ASE behavior of three polymers, together with the output intensity and FWHM versus pump energy density. As a consequent of similar mainchain, three polymers had a similar ASE peak at around 463~468 nm, detailed as 465 nm for PEODPF, 463 nm for POYDPF and 468 nm for PODPF (Figs. 6a-c), attributed to the 0-1 vibration transition of mainchain. Interestingly, ASE threshold of PEODPF, POYDPF and PODPF are estimated about 6.70 μJ/cm2, 5.45 μJ/cm2 and 4.72 μJ/cm2 (Figs. 6d-f), respectively, which are a low threshold value compared to the reported deep-blue light-emitting conjugated polymer. In this regard, there is slightly branched effect on the ASE behavior of pristine spincoated films. Meanwhile, we also measured the ASE behavior of annealed film to check corresponding thermal stability of the gain property. As shown in Fig. 6, ASE peak of PEODPF and POYDPF annealed films are also emerged at 463 and 462 nm, similar to the pristine ones, associated with the 0-1 emission peak. These results also confirmed the excellent spectral stability upon thermal annealing at 220 ℃, similar to the analysis result of AFM images. However, as a result of the phase and conformation transition, PODPF annealed films presented an ASE peak at 484 nm, attributed the 0-1 emission peak of β-conformation, consisted with the result of optical analysis. As we expected, the ASE threshold of the PEODPF and POYDPF annealed films are 5.53 μJ/cm2, 5.35 μJ/cm2, similar to the pristine ones, also confirmed the excellent spectral morphological stability. However, upon thermal annealing at 220 ℃, it is easy to find that the ASE threshold of PODPF annealed films are increased dramatically, attributed to rough morphology and serious crystallization (Fig. 2d). Therefore, this branch effect enables PEODPF and POYDPF film to obtain excellent stable and low threshold ASE behavior for organic laser, attributed to the suppression of phase/conformational transition.

|
Download:
|
| Fig. 6. ASE behaviour of three polymer pristine and annealed films. | |
To summarize, we systematically demonstrate the effect of branched effect of the side chain on interchain aggregation and photophysical behaviour of polydiarylfluorenes. Compared to the liner type PODPF, the corporation of branched side chain can not only effectively suppress planar conformation transition and enhance morphological stability, but also improve solubility to improve film-forming ability. Therefore, our branched polydiaryl-fluorenes, PEODPF and POYDPF, exhibited robust deep-blue emission and relatively stable film morphology upon thermal annealing at 220 ℃, compared to PODPF ones. Besides, PEODPF and POYDPF also show stable deep-blue EL spectra with single-chain excitonic behavior. Finally these robust emission and stable film morphology also enable branched polydiarylfluorenes to present low-threshold and efficient deep-blue ASE behavior. Therefore, this branched strategy of side chain will inspire widely attention to design high performance light-emitting conjugated polymer.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 61874053, 21774061, 91833306), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, PAPD (No. YX03002), the Six Peak Talents Foundation of Jiangsu Province (No. XCL-CXTD-009), Natural Science Funds of the Education Committee of Jiangsu Province (No.18KJA430009), "High-Level Talents in Six Industries" of Jiangsu Province (No. XYDXX-019), and Program for Postgraduates Research Innovation in University of Jiangsu Province (No. KYCX17_0752), the open research fund from Key Laboratory for Organic Electronics and Information Display & and State Key Laboratory of Supramolecular Structure and Materials (No. sklssm2019017), Overseas Merit Foundation of Science and Technology of Nanjing. Juan Cabanillas-Gonzalez acknowledges financial support from the Regional Government of Madrid through NMAT2D-CM Project (No. S2018/NMT-4511) and from the Spanish Ministry of Economy and Competitiveness through project RTI2018-097508-B-I00 and through the Severo Ochoa Program for Centers of Excellence (No. SEV-2016-0686) and the Campus of International Excellence (CEI) UAM+CSIC. Juan Cabanillas-Gonzalez and Chen Sun are also grateful to the China Scholarship Council (No. 201608390023) for a PhD sponsorship.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.08.048.
| [1] |
X.H. Jin, M.B. Price, J.R. Finnegan, et al., Science 360 (2018) 897-900. DOI:10.1126/science.aar8104 |
| [2] |
J.Y. Oh, S Rondeau-Gagné, Y.C. Chiu, et al., Nature 539 (2016) 411. DOI:10.1038/nature20102 |
| [3] |
J. Zhao, Y. Li, G. Yang, et al., Nat. Energy 1 (2016) 15027. DOI:10.1038/nenergy.2015.27 |
| [4] |
K. Bong-Gi, J. Eun Jeong, C. Jong Won, et al., Nat. Mater. 12 (2013) 659-664. DOI:10.1038/nmat3595 |
| [5] |
Y. Boon Kar, X. Ruidong, C.Q. Mariano, et al., Nat. Mater. 7 (2008) 376-380. DOI:10.1038/nmat2165 |
| [6] |
G. Li, R. Zhu, Y. Yang, Nat. Photonics 6 (2012) 153. DOI:10.1038/nphoton.2012.11 |
| [7] |
J. Lin, B. Liu, M. Yu, et al., Adv. Mater. 1 (2019) 1804811. |
| [8] |
A.J. Heeger, Chem. Soc. Rev. 39 (2010) 2354-2371. DOI:10.1039/b914956m |
| [9] |
C. Yan, S. Barlow, Z. Wang, et al., Nat. Rev. Mater. 3 (2018) 18003. DOI:10.1038/natrevmats.2018.3 |
| [10] |
L.H. Xie, C.R. Yin, W.Y. Lai, et al., Prog. Polym. Sci. 37 (2012) 1192-1264. DOI:10.1016/j.progpolymsci.2012.02.003 |
| [11] |
J. Yuan, Y. Zhang, L. Zhou, et al., Joule 3 (2019) 1140-1151. DOI:10.1016/j.joule.2019.01.004 |
| [12] |
Y. Liu, J. Zhao, Z. Li, et al., Nat. Commun. 5 (2014) 5293. DOI:10.1038/ncomms6293 |
| [13] |
A. Hayer, A.L.T. Khan, R.H. Friend, et al., Phys. Rev. B 71 (2005) 241302. DOI:10.1103/PhysRevB.71.241302 |
| [14] |
A.L.T. Khan, P. Sreearunothai, L.M. Herz, et al., Phys. Rev. B 69 (2004) 85201. DOI:10.1103/PhysRevB.69.085201 |
| [15] |
F.B.D. Dr, K.T.K. Dr, T.C. Dr, et al., Chemphyschem. A Eur. J. Chem. Phys. Phys. Chem 10 (2010) 2096-2104. |
| [16] |
K. Matti, A.P. Monkman, Adv. Mater. 25 (2013) 1090-1108. DOI:10.1002/adma.201204296 |
| [17] |
C. Rothe, F. Galbrecht, U. Scherf, et al., Adv. Mater. 18 (2006) 2137-2140. DOI:10.1002/adma.200600901 |
| [18] |
A. Perevedentsev, Y. Sonnefraud, C.R. Belton, et al., Nat. Commun. 6 (2015) 5977. DOI:10.1038/ncomms6977 |
| [19] |
M.N. Yu, B. Liu, J.Y. Lin, et al., Chin. J. Polym. Sci. 34 (2016) 1311-1318. DOI:10.1007/s10118-016-1851-z |
| [20] |
Z.Q. Lin, N.E. Shi, Y.B. Li, et al., J. Phys. Chem. C 115 (2011) 4418-4424. DOI:10.1021/jp109598y |
| [21] |
D.W. Bright, F.B. Dias, F. Galbrecht, et al., Adv. Funct. Mater. 19 (2010) 67-73. |
| [22] |
A.T. Yiu, P.M. Beaujuge, O.P. Lee, et al., J. Am. Chem. Soc. 134 (2012) 2180-2185. DOI:10.1021/ja2089662 |
| [23] |
M. Jianguo, K.D. Hwan, A.L. Ayzner, et al., J. Am. Chem. Soc. 133 (2011) 20130-20133. DOI:10.1021/ja209328m |
| [24] |
L. Junghoon, H. A-Reum, K. Jonggi, et al., J. Am. Chem. Soc. 134 (2012) 20713-20721. DOI:10.1021/ja308927g |
| [25] |
J. Mei, Z. Bao, Chem. Mater. 26 (2014) 604-615. DOI:10.1021/cm4020805 |
| [26] |
B. Kang, K. Ran, S.B. Lee, J. Am. Chem. Soc. 138 (2016) 3679-3686. DOI:10.1021/jacs.5b10445 |
| [27] |
S. Inoue, H. Minemawari, J.Y. Tsutsumi, et al., Chem. Mater. 27 (2015) 3809-3812. DOI:10.1021/acs.chemmater.5b00810 |
| [28] |
Y.J. Cheng, S.H. Yang, C.S. Hsu, Chem. Rev. 109 (2009) 5868-5923. DOI:10.1021/cr900182s |
| [29] |
X. Zhang, Q. Hu, J. Lin, et al., Appl. Phys. Lett. 103 (2013) 153301. DOI:10.1063/1.4824766 |
| [30] |
L.B. Bai, B. Liu, Y.M. Han, et al., ACS Appl. Mater. Interfaces 9 (2017) 37856-37863. DOI:10.1021/acsami.7b08980 |
| [31] |
F.B. Dias, J. Morgado, A.L. Maçanita, et al., Macromolecules 39 (2006) 5854-5859. DOI:10.1021/ma0602932 |
| [32] |
B. Liu, J. Lin, F. Liu, et al., ACS Appl. Mater. Interfaces 8 (2016) 21648-21655. DOI:10.1021/acsami.6b05247 |
| [33] |
B. Liu, J.Y. Lin, M.N. Yu, et al., J. Phys. Chem. C 121 (2017) 19087-19096. DOI:10.1021/acs.jpcc.7b06330 |
| [34] |
A. Perevedentsev, P.N. Stavrinou, D.D.C. Bradley, et al., J. Polym. Sci. Part B:Polym. Phys. 53 (2015) 1481-1491. DOI:10.1002/polb.23798 |
| [35] |
J.H. Chen, C.S. Chang, Y.X. Chang, et al., Macromolecules 42 (2009) 1306-1314. DOI:10.1021/ma802408u |
| [36] |
B. Liu, L. Tao, H. Zhang, et al., J. Phys. Chem. C 122 (2018) 14814-14826. DOI:10.1021/acs.jpcc.8b03504 |
| [37] |
H. Ling, J. Lin, M. Yi, et al., ACS Appl. Mater. Interfaces 8 (2016) 18969-18977. DOI:10.1021/acsami.6b03792 |
| [38] |
M.N. Yu, H. Soleimaninejad, J.Y. Lin, et al., J. Phys. Chem. Lett. 9 (2018) 364-372. DOI:10.1021/acs.jpclett.7b03148 |
| [39] |
B. Liu, J. Lin, Z. Lei, et al., Macromol. Chem. Phys. 216 (2015) 1043-1054. DOI:10.1002/macp.201400568 |
| [40] |
J.Y. Lin, W.S. Zhu, F. Liu, et al., Macromolecules 47 (2014) 1001-1007. DOI:10.1021/ma402585n |
| [41] |
W. Xue, J.Y. Lin, B. Liu, et al., Polymer 153 (2018) 338-343. DOI:10.1016/j.polymer.2018.05.025 |
| [42] |
T. Lei, J.H. Dou, J. Pei, Adv. Mater. 24 (2012) 6457-6461. DOI:10.1002/adma.201202689 |
| [43] |
Y.M. Han, L.B. Bai, C.J. Ou, et al., J. Mater. Chem. C 5 (2017) 9903-9910. DOI:10.1039/C7TC03536E |
| [44] |
C. Franco, J.S. Wilson, J.J. Michels, et al., Nat. Mater. 1 (2002) 160-164. DOI:10.1038/nmat750 |
| [45] |
J. Lin, Z. Yu, W. Zhu, et al., Polym. Chem. 4 (2013) 477-483. DOI:10.1039/C2PY20618H |
 2019, Vol. 30
2019, Vol. 30