Theranostic is defined as a material that combines both diagnostic and therapeutic functions [1-5]. Molecularly theranostics based strategy can simultaneously realize the therapy with diagnostic information in a single-entity platform and evaluate in real-time the prognosis after therapy [6-19]. Indeed, molecular imaging serves this diagnostic function and provides powerful means for specific molecular information on the presence of defined molecular targets before, during, and after therapy [20]. There has been an explosive development in the design of molecular imaging contrasts and imaging-guided therapeutics [21]. To date, a number of near-infrared (NIR) fluorescent probes have been developed for tracing molecular processes in vitro and in vivo [22-25]. In particular, molecularly NIR fluorescent prodrugs that enabling in vivo imaging and therapeutic chemotherapy (sense-and-release) have received considerable attention to diagnose and treat cancer [26].
NIR fluorescence light (650–900 nm) has been widely utilized in clinical imaging by providing surgeons highly specific images of target tissue [27-31]. Compared with the visible emission, NIR emission possesses unique advantages including minimized interfere of auto-fluorescence, deep tissues penetration, and less damage to biological samples [32-36]. In molecular theranostics, NIR fluorescence is a preferential way for real-time tracking senseand-release, enabling intelligent recognition and then releasing optimal dosages of anticancer drugs for specific therapy.
In this review, we summarized the design strategies of NIR fluorescent theranostics prodrugs for the sense-and-release of tumor-specific chemotherapy in Scheme 1, including OFF-ON pattern based NIR theranostics, dual-channel pattern based NIR theranostics, physically encapsulated NIR theranostics with nanocarriers, molecularly precise self-assembly of NIR theranostics, and sense-of-logic NIR theranostic prodrug. Attention is given to contributions during the period 2014–2019 and focused on molecularly NIR fluorescent theranostic prodrug for bio-imaging and targeted therapy in vitro and in vivo. In addition, the sensing mechanisms and interaction modes of representative NIR theranostics prodrugs are also discussed.
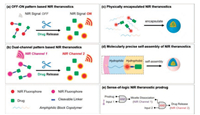
|
Download:
|
| Scheme 1. Strategies of molecularly NIR theranostics for real-time tracking chemotherapy. | |
2. OFF-ON pattern based NIR theranostics
In situ tracking of prodrugs after in vivo uptake, particularly in a non-invasive manner, is of critical importance. Notably, equipping with high-performance fluorophores as optical reporters have become an attractive strategy for monitoring the drug delivery and release process However, owing to poor photo-stability of NIR fluorescent reporters, there is still a lack of in vivo and in situ tracking of the drug release. As typical donor-π-acceptor (D-π-A) structured chromophores, dicyanomethylene-4H-pyran (DCM) derivatives display a broad absorption band resulting from an ultra-fast internal charge-transfer (ICT) process [37-42]. In recent years, various DCM-type derivatives have been explored for developing NIR emission chemosensors because of their controllable emission wavelength in the NIR region via tuning electron donor ability, large Stokes shift from the ICT process, and high photostability [43-47]. Specifically, these excellent properties make DCM-derivatives ideally suitable for constructing an OFF-ON emission pattern of NIR fluorescent theranostic prodrug (Scheme 1a).
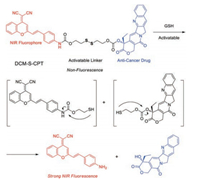
In 2014, Zhu et al. described a GSH-activatable NIR theranostic strategy that allows directly in vivo and in situ monitoring of cancer chemotherapy (Fig. 1) [48]. DCM-S-CPT as an activatable nonfluorescent prodrug, is composed of DCM unit as the NIR fluorescent reporter with high photostability and camptothecin (CPT) as the anticancer drug, which are linked by a disulfide linker. Upon reaction with reducing biothiols, the cleavage of disulfide bond occurred, resulting in concomitantly the CPT release and the generation of turn-on NIR fluorescence at 660 nm. In vitro experiments further verified that the high endogenous glutathione (GSH) concentrations in cancer MCF-7 cells could cause the intracellular cleavage of disulfide linkage, resulting in the active CPT release with significant turn-on NIR fluorescence for monitoring the drug release profiles. Impressively, all the results of in vitro and in vivo experiments show that DCM-S-CPT could be used for in vivo tracking of drug release and cancer therapeutic efficacy in living animals by the NIR fluorescence. Notably, its OFFON pattern of NIR wavelength and high photo-stability makes DCM-S-CPTas a promising prodrug towards deeper understanding and exploring theranostic drug-delivery systems.
|
Download:
|
| Fig. 1. Proposed GSH sensing and drug release mechanism of DCM-S-CPT. Reproduced with permission [48]. Copyright 2014, American Chemical Society.. | |
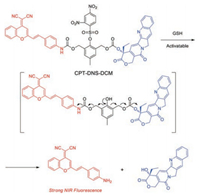
In 2017, Wu et al. reporteda DCM-based activatable NIR prodrug (CPT-DNS-DCM) (Fig. 2) [49]. A GSH-triggered and self-immolative dendritic unit was bilaterally modified CPT and DCM unit. In the prodrug CPT-DNS-DCM, the initial fluorescence of DCM fluorophore is almost completely quenched by the electron-withdrawing self-immolative unit. In the presence of abundant GSH, the cascade of reaction is triggered with the release of active CPTand switching on NIR emission for two-photon bioimaging in HeLa cells. The prodrug is also utilized for in vivo tracking the drug release in tumor-bearing mice. Furthermore, CPT-DNS-DCM exhibits high inhibition on tumor growth from in situ antitumor test.
|
Download:
|
| Fig. 2. Proposed GSH sensing and drug release mechanism of CPT-DNS-DCM. Reproduced with permission [49]. Copyright 2017, Elsevier. | |
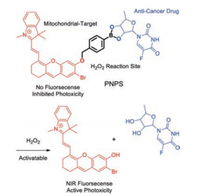
The merocyanine fluorophore is another good candidate of signal transducers for chemosensors because of their excellent photophysical properties and low toxicity to living systems. A tremendous effort based on the merocyanine platform have devoted to the development of chemosensors in recent years [50-70]. In 2017, Liu et al. reported an activatable merocyaninebased mitochondrial targeting prodrug (PNPS) for fluorescence imaging-guided and synergetic chemo-photodynamic therapy (Fig. 3) [71]. The introduced merocyanine unit with bromide atom is employed as both the NIR optical reporter and photosensitizer, 50'DFUR as an anticancer drug, wherein these two parts are linked via a bisboronate group (a H2O2-sensitive unit). The NIR fluorescence at 710 nm from merocyanine unit is completely quenched when it is covalently linked to the bisboronate group. The prodrug PNPS is activated for the sense-release by the high level of H2O2, leading to an obvious enhancement of NIR emission for real-time tracking the drug release in living cells and mice. Notably, owing to the chemo-photodynamic therapy and effective mitochondrial targeting ability, PNPS displays enhanced therapy efficiency compared to only free drugs.
|
Download:
|
| Fig. 3. Proposed sensing and release mechanism of PNPS. Reproduced with permission [71]. Copyright 2017, Royal Society of Chemistry. | |
Heptamethine cyanines (Cy7) are one of typical NIR fluorescent dyes for tumor imaging because they can emit light in the NIR region, which is capable of deep tissue penetration and minimized autofluorescence of tissue [72-97]. In 2014, Yang et al. designed and synthesized a series of Cy7-based prodrugs using disulfide group as GSH-activatable unit, folate group as tumor-targeting ligand, and gemcitabine as anticancer drug (prodrug-2, Fig. 4) [98]. Upon the disulfide bond cleavage with GSH, the amide group could be rapidly decomposed into an amine group, leading to the release of active gemcitabine and a sharply NIR fluorescence enhancement at 735 nm. With the help of the OFF-ON NIR response, in vivo fluorescence imaging experimentation upon intravenous injection confirmed that the prodrug was selectively taken up by KB over A549 tumor tissue and its active gemcitabine was released.
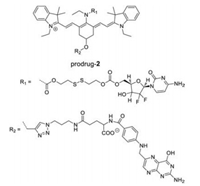
|
Download:
|
| Fig. 4. Proposed GSH sensing and drug release mechanism of prodrug-2 Reproduced with permission [98]. Copyright 2013, American Chemical Society. | |
3. Dual-channel pattern based NIR theranostics
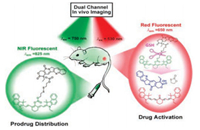
It is worth noting that most of currently NIR fluorescent prodrugs suffered from only one-channel turn-on emission. In this regard, the in vivo biodistribution of prodrugs before activation in a certain organ or tissue become blind. In 2016, Zhu et al. designed and synthesized a dual-channel NIR fluorescence activatable theranostic prodrug for real-time tracking where, when, and how (WWH) prodrugs are delivered and activated (Scheme 1b) [99]. The cyanine moiety and CPT are covalently bridged with the disulfide bond via carbonate bonds (Cy-S-CPT) (Fig. 5).
|
Download:
|
| Fig. 5. Proposed GSH sensing (dual-channel response) and drug release mechanism of Cy-S-CPT. Copied with permission [99]. Copyright 2016, Royal Society of Chemistry. | |
Indeed, the specific cleavage of the disulfide bond in Cy-S-CPT by glutathione (GSH) and the successive cyclization produces the activated CPT and induces a remarkable blue shift from 825 nm to 650 nm in emission spectra. The distinct spectral changes could be due to the disruption in the polymethine π-electron system after the reaction with GSH, so that the π-conjugation system in the tricarbocyanine chromophore was obviously shortened. These dual-channel emission responses are utilized to real-timely scrutinize the activation process of prodrugs with disulfide bond linker in vivo : the 825 nm-NIR fluorescence channel for tracking the biodistribution of the intact prodrug, while the 650 nm red fluorescence channel for monitoring the activated drug. The dualchannel in vivo pharmacokinetic and biodistribution study (in BCap-37 tumor-bearing mice) indicates that the prodrug could be activated not only in the tumors, but also in the other organs, particularly the liver.
Remotely controlled (for example light-mediated manner) drug delivery is also an effective strategy for precise diagnosis and therapy in cancer treatment. Optical orthogonality that conjuncts with light-trigger and in vivo NIR fluorescence bioimaging could guarantee the drug release at the right place and time, wherein the in vivo behavior of these prodrugs is established and site-activated release is regulated for improving therapeutics. In general, it is difficult to balance the dosimetry of drug with light dose and a lack of in vivo models for validating their clinical benefits. Recently, Guo et al. reported a dual-channel NIR photocaged prodrug (Cy-CPTBiotin), wherein a dialkylamine-substituted tricarboncyanine as light-responsive core was covalently bridged with CPT via carbamate bonds (Fig. 6) [100]. Upon NIR-light irradiation, its polyene bond could be broken to form with new-channel emission (from 820 nm to 535 nm) and concurrently leading to active CPT release. The in vivo and ex vivo imaging of tumor-bearing mice indicated that the NIR fluorescent signal could be serviced for real-time tracing where the intact prodrugs are located in vivo, and this signal could further visually guide the spatial light irradiation.

|
Download:
|
| Fig. 6. Illustration of NIR light-mediated dual-channel fluorescent prodrug Cy-CPT-Biotin for precise cancer diagnosis and therapy. Reproduced with permission [100]. Copyright 2018, Science China Press. | |
4. Physically encapsulated NIR theranostics
The creation of nanotheranostics based on molecular prodrugs can promise such immense benefits for targeted therapies. Most of current drug delivery strategies mainly focus on physical entrapment, including polymers, liposomes and inorganic materials. With the help of these nanovesicles, drugs with prolonged blood circulation duration and enhanced permeability and retention (EPR effect) show more effective and specific cancer treatment than free drugs (Scheme 1c) [101-104]. Liu et al. described the synthesis and biological assessment of the prodrug DCM-S-PPTand constructed amphiphilic copolymer-encapsulated nanotheranostics (Fig. 7) [105]. In their work, podophyllotoxin (PPT) was employed as the anti-cancer drug, DCM unit as the NIR fluorescent reporter, and disulfide bond as the cleavable linker. The drug release mechanism of DCM-S-PPT is very similar to the reported DCM-S-CPT (Fig. 1): GSH triggered cleavage of the disulfide linker leading to the PPT release and significant NIR fluorescence off-on response. With the help of mPEG-DSPE as a suitable nanocarrier, the obtained mPEG-DSPE/DCM-S-PPT displays much better tumortargeting ability than that of free DCM-S-PPT on account of the enhanced permeability and EPR effect.

|
Download:
|
| Fig. 7. Chemical structure of DCM-S-PPT and its amphiphilic copolymer-encapsulated nanotheranostics. Reproduced with permission [105]. Copyright 2017, American Chemical Society. | |
Recently, Zhu et al. reported a NIR fluorescent prodrug and its amphiphilic copolymer-encapsulated nanotheranostics (DCM-SPt@PEG) for transcatheter intra-arterial therapy towards rabbit hepatocellular carcinoma (Fig. 8) [106]. DCM-S-Pt contains three functional components: an optical reporter NIR DCM moiety, an antitumor drug Pt(II) cisplatin, and a disulfide linker for specific activation by GSH. With the help of transcatheter intra-arterial therapy method and DSPE-mPEG (nanocarrier), DCM-S-Pt@PEG displays well controllable drug release to avoid fast metabolism, and high efficient tumor growth inhibition in hepatocellular carcinoma rabbit. For the first time, this work illustrates how to achieve direct fluorescent visualization, increase tumor targeting ability, and well control of the sustained release of cisplatin for hepatocellular carcinoma treatment on large mammal rabbit model with transcatheter intra-arterial therapy.
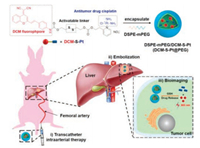
|
Download:
|
| Fig. 8. Chemical structure of DCM-S-Pt and its amphiphilic copolymer-encapsulated nanotheranostics. Reproduced with permission [106]. Copyright 2018, Wiley Publishing Group. | |
5. Molecularly precise self-assembly of NIR theranostics
Small molecule fluorescent prodrugs loaded in nanocarriers have passive tumor-targeting ability (EPR effect) as promising theranostics and perform the activatable release in tumors [107]. However, structural heterogeneity, inevitable leakage and nonuniform loading efficiency are insuperable barriers. To address these hurdles, the design of monodisperse nanomaterials with a single, reproducible entity that possesses both in vivo diagnostic and therapeutic competencies is in demand (Scheme 1d).
Recently, Yan et al. described the strategy of molecularly precise self-assembly of monodisperse nanotheranostics for BPn-DCM-SCPT (n = 0, 5 and 20) with fixed drug loadings, permitting in vivo real-time targeted chemotherapy (Fig. 9) [108]. Taking the biscondensed DCM derivative as the activatable NIR fluorophore, it made use of two-terminal conjunctions: the hydrophobic disulfide-bridged anticancer prodrug CPT and the hydrophilic oligomer bridged biotin segment serving as an active targeting unit. In aqueous solution, BP20-DCM-S-CPT could spontaneously form uniform and highly stable self-assemblies (ca. 80 nm, critical micelle concentration =1.52 μmol/L), while not for BP5-DCM-SCPT and DCM-S-CPT. The in vivo imaging and anti-tumor experiments confirm that BP20-DCM-S-CPT performs excellent tumor-specific drug release in HeLa tumor-bearing nude mice, which had significantly enhanced in vivo antitumor activity and nearly eradicated the tumor (IRT = 99.7%) with few side effects.
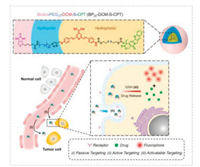
|
Download:
|
| Fig. 9. Amphiphilic self-assembled nanotheranostic systems. Tumor-specific molecularly precise self-assembled nanotheranostics for BP20-DCM-S-CPT with enhanced synergistic targeting. Reproduced with permission [108]. Copyright 2018, Royal Society of Chemistry. | |
6. Sense-of-logic NIR theranostic prodrug
Current multi-stimulus-responsive probes are predominantly operated by "OR" logic gates in response to each stimulus. However, nonspecific activation in complex biological milieu always leads to "false positive" signals with difficulty in accurate recognition. For precisely controlling the drug release in vivo, Yan et al. presented a proof-of-concept study of a sequence activated AND logic dual-channel NIR fluorescent probe P(Cy-S-CPT) (Scheme 1e and Fig. 10), which functions as a programmable sensor and then releases anticancer drugs [109]. The smart nanoprobe P(Cy-S-CPT) is composed of two functional components: an ionizable tertiary amine-containing diblock copolymer which rendered an ultra-sensitive response to small pH differences between acidic tumor cells and blood, and a dual-channel NIR fluorescence component Cy-S-CPT for tracking the biothioltriggered prodrug release in vivo. With the stimuli of pH and biothiols, sequence-dependent dual-channel NIR fluorescence output is in synchronism with the programmable drug release. An unprecedented integration of functional sense-and-release makes a breakthrough to real-time tracking of each step that lead to in drug release, along with three-dimensional bioimaging from dual-channel NIR fluorescence feedbacks. The significant enhancement in antitumor activity in vivo demonstrated that P(Cy-S-CPT) promote the therapeutic efficiency via excellent multistaged tumor targeting ability and then nearly eradicate the tumor with the high inhibition (IRT = 93.6%).

|
Download:
|
| Fig. 10. Illustration of sequence-activated AND logic dual-channel NIR fluorescent probe. Reproduced with permission [109]. Copyright 2018, Royal Society of Chemistry. | |
7. Conclusions and perspectives
In this review, recent advances (2014–2019) made in the study NIR fluorescent theranostics prodrugs were overviewed. Particular attention was focused on how the utilization of NIR fluorescent probes for in vivo sense-and-release in the design of molecular theranostics. Despite tremendous advances in cancer therapy, the integration of multimodality diagnostic into a single-entity of molecular thernanostics remains great challenges for in vivo tracking tumor-specific chemotherapy. For instance, it is still difficult to assess in vivo quantitation of dosage of the released drug. In addition, it is also expected to develop theranostic prodrugs with expanded emission wavelength (NIR-II region) for deep-tissue applications. Clearly, devising general and effective approaches to versatile theranostics prodrugs for personalized treatment will be the impetus for future developing exciting new methods, aiming at molecular multimode imaging with in vivo quantitative sense-and-release.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21788102, 21421004, 21636002, 21622602 and 21908060), National Key Research and Development Program (No. 2017YFC0906902), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX03), the Innovation Program of Shanghai Municipal Education Commission, Scientific Committee of Shanghai (No. 15XD1501400), and China Postdoctoral Science Foundation (No. 2019M651417).
| [1] |
M.H. Lee, J.L. Sessler, J.S. Kim, Acc. Chem. Res. 48 (2015) 2935-2946. DOI:10.1021/acs.accounts.5b00406 |
| [2] |
M.H. Lee, Z. Yang, C.W. Lim, et al., Chem. Rev. 113 (2013) 5071-5109. DOI:10.1021/cr300358b |
| [3] |
R. Kumar, W.S. Shin, K. Sunwoo, et al., Chem. Soc. Rev. 44 (2015) 6670-6683. DOI:10.1039/C5CS00224A |
| [4] |
M.H. Lee, A. Sharma, M.J. Chang, et al., Chem. Soc. Rev. 47 (2018) 28-52. DOI:10.1039/C7CS00557A |
| [5] |
X. Li, S. Lee, J. Yoon, Chem. Soc. Rev. 47 (2018) 1174-1188. DOI:10.1039/C7CS00594F |
| [6] |
C. Chen, H. Ou, R. Liu, D. Ding, Adv. Mater. 31 (2019) 806331. |
| [7] |
B. Gu, W. Wu, G. Xu, et al., Adv. Mater. 29 (2017) 1701076. DOI:10.1002/adma.201701076 |
| [8] |
F. Hu, S. Xu, B. Liu, Adv. Mater. 30 (2018) 1801350. DOI:10.1002/adma.201801350 |
| [9] |
D. Li, W. Qin, B. Xu, J. Qian, B.Z. Tang, Adv. Mater. 29 (2017) 1703643. DOI:10.1002/adma.201703643 |
| [10] |
S. Bhuniya, S. Maiti, E.J. Kim, et al., Angew. Chem. Int. Ed. 53 (2014) 4469-4474. DOI:10.1002/anie.201311133 |
| [11] |
X. Li, N. Kwon, T. Guo, Z. Liu, J. Yoon, Angew. Chem. Int. Ed. 57 (2018) 11522-11531. DOI:10.1002/anie.201805138 |
| [12] |
X. Li, D. Lee, J.D. Huang, J. Yoon, Angew. Chem. Int. Ed. 57 (2018) 9885-9890. DOI:10.1002/anie.201806551 |
| [13] |
A. Sharma, M.G. Lee, H. Shi, et al., Chem 4 (2018) 2370-2383. DOI:10.1016/j.chempr.2018.08.002 |
| [14] |
A. Sharma, J.F. Arambula, S. Koo, et al., Chem. Soc. Rev. 48 (2019) 771-813. DOI:10.1039/C8CS00304A |
| [15] |
H.S. Jung, J. Han, H. Shi, et al., J. Am. Chem. Soc. 139 (2017) 7595-7602. DOI:10.1021/jacs.7b02396 |
| [16] |
H.S. Jung, J.H. Lee, K. Kim, et al., J. Am. Chem. Soc. 139 (2017) 9972-9978. DOI:10.1021/jacs.7b04263 |
| [17] |
M.H. Lee, E.J. Kim, H. Lee, et al., J. Am. Chem. Soc. 138 (2016) 16380-16387. DOI:10.1021/jacs.6b09713 |
| [18] |
S. Liu, X. Zhou, H. Zhang, et al., J. Am. Chem. Soc. 141 (2019) 5359-5368. DOI:10.1021/jacs.8b13889 |
| [19] |
L. Xu, L. Jiang, M. Drechsler, et al., J. Am. Chem. Soc. 136 (2014) 1942-1947. DOI:10.1021/ja410443n |
| [20] |
X. Xu, P.E. Saw, et al., Adv. Mater. 29 (2017) 1700141. DOI:10.1002/adma.201700141 |
| [21] |
F. Hu, D. Mao, Kenry, et al., Angew. Chem. Int. Ed. 57 (2018) 10182-10186. DOI:10.1002/anie.201805446 |
| [22] |
K.G. Chernov, T.A. Redchuk, E.S. Omelina, V.V. Verkhusha, Chem. Rev. 117 (2017) 6423-6446. DOI:10.1021/acs.chemrev.6b00700 |
| [23] |
Y. Ding, W.H. Zhu, Y. Xie, Chem. Rev. 117 (2017) 2203-2256. DOI:10.1021/acs.chemrev.6b00021 |
| [24] |
Y. Cai, Z. Wei, C. Song, et al., Chem. Soc. Rev. 48 (2019) 22-37. DOI:10.1039/C8CS00494C |
| [25] |
F. Deng, Z. Xu, Chin. Chem. Lett. (2018), doi: http://dx.doi.org/10.1016/j.cclet.2018.12.012.
|
| [26] |
A.P. Gorka, R.R. Nani, M.J. Schnermann, Acc. Chem. Res. 51 (2018) 3226-3235. DOI:10.1021/acs.accounts.8b00384 |
| [27] |
H. Chen, B. Dong, Y. Tang, W. Lin, Acc. Chem. Res. 50 (2017) 1410-1422. DOI:10.1021/acs.accounts.7b00087 |
| [28] |
Y. Jiang, K. Pu, Acc. Chem. Res. 51 (2018) 1840-1849. DOI:10.1021/acs.accounts.8b00242 |
| [29] |
E.A. Owens, M. Henary, G. El Fakhri, H.S. Choi, Acc. Chem. Res. 49 (2016) 1731-1740. DOI:10.1021/acs.accounts.6b00239 |
| [30] |
S. He, J. Song, J. Qu, Z. Cheng, Chem. Soc. Rev. 47 (2018) 4258-4278. DOI:10.1039/C8CS00234G |
| [31] |
X. Luo, J. Li, J. Zhao, et al., Chin. Chem. Lett. 30 (2019) 839-846. DOI:10.1016/j.cclet.2019.03.012 |
| [32] |
H.J. Knox, J. Chan, Acc. Chem. Res. 51 (2018) 2897-2905. DOI:10.1021/acs.accounts.8b00351 |
| [33] |
Q. Miao, K. Pu, Adv. Mater. 30 (2018) 1801778. DOI:10.1002/adma.201801778 |
| [34] |
L. Wang, W. Du, Z. Hu, et al., Angew. Chem. Int. Ed. (2019), doi: http://dx.doi.org/10.1002/anie.201901061.
|
| [35] |
Z. Guo, S. Park, J. Yoon, I. Shin, Chem. Soc. Rev. 43 (2014) 16-29. DOI:10.1039/C3CS60271K |
| [36] |
M. Montalti, A. Cantelli, G. Battistelli, Chem. Soc. Rev. 44 (2015) 4853-4921. DOI:10.1039/C4CS00486H |
| [37] |
S.M. Fateminia, Z. Wang, C.C. Goh, et al., Adv. Mater. 29 (2017) 1604100. DOI:10.1002/adma.201604100 |
| [38] |
X. Jia, Y. Zhang, Y. Zou, et al., Adv. Mater. 30 (2018) 1704490. DOI:10.1002/adma.201704490 |
| [39] |
D. Venkatakrishnarao, Y.S. Narayana, M.A. Mohaiddon, et al., Adv. Mater. 29 (2017) 1605260. http://www.ncbi.nlm.nih.gov/pubmed/28112830/
|
| [40] |
Z. Guo, W. Zhu, L. Shen, H. Tian, Angew. Chem. Int. Ed. 46 (2007) 5549-5553. DOI:10.1002/anie.200700526 |
| [41] |
A. Shao, Y. Xie, S. Zhu, et al., Angew. Chem. Int. Ed. 54 (2015) 7275-7280. DOI:10.1002/anie.201501478 |
| [42] |
Z. Guo, W. Zhu, H. Tian, Chem. Comm. 48 (2012) 6073-6084. DOI:10.1039/c2cc31581e |
| [43] |
W. Sun, J. Fan, C. Hu, et al., Chem. Comm. 49 (2013) 3890-3892. DOI:10.1039/c3cc41244j |
| [44] |
W. Zhu, X. Huang, Z. Guo, et al., Chem. Comm. 48 (2012) 1784-1786. DOI:10.1039/c2cc16902a |
| [45] |
H.N. Kim, Z. Guo, W. Zhu, J. Yoon, H. Tian, Chem. Soc. Rev. 40 (2011) 79-93. DOI:10.1039/C0CS00058B |
| [46] |
W. Fu, C. Yan, Z. Guo, et al., J. Am. Chem. Soc. 141 (2019) 3171-3177. DOI:10.1021/jacs.8b12820 |
| [47] |
K. Gu, Y. Xu, H. Li, et al., J. Am. Chem. Soc. 138 (2016) 5334-5340. DOI:10.1021/jacs.6b01705 |
| [48] |
X. Wu, X. Sun, Z. Guo, et al., J. Am. Chem. Soc. 136 (2014) 3579-3588. DOI:10.1021/ja412380j |
| [49] |
Z. Wang, H. Wu, P. Liu, F. Zeng, S. Wu, Biomaterials 139 (2017) 139-150. DOI:10.1016/j.biomaterials.2017.06.002 |
| [50] |
Y. Liu, Q. Su, M. Chen, et al., Adv. Mater. 28 (2016) 6625-6630. DOI:10.1002/adma.201601140 |
| [51] |
Q. Gong, R. Zou, J. Xing, et al., Adv. Sci. 5 (2018) 1700664.
|
| [52] |
Y. Liu, J. Niu, W. Wang, Y. Ma, W. Lin, Adv. Sci. 5 (2018) 1700966. DOI:10.1002/advs.201700966 |
| [53] |
Q. Miao, D.C. Yeo, C. Wiraja, et al., Angew. Chem. Int. Ed. 57 (2018) 1256-1260. DOI:10.1002/anie.201710727 |
| [54] |
Q. Wan, S. Chen, W. Shi, L. Li, H. Ma, Angew. Chem. Int. Ed. 53 (2014) 10916-10920. DOI:10.1002/anie.201405742 |
| [55] |
X. Wu, L. Li, W. Shi, Q. Gong, H. Ma, Angew. Chem. Int. Ed. 55 (2016) 14728-14732. DOI:10.1002/anie.201609895 |
| [56] |
G. Xu, Q. Yan, X. Lv, et al., Angew. Chem. Int. Ed. 57 (2018) 3626-3630. DOI:10.1002/anie.201712528 |
| [57] |
X. Zhen, J. Zhang, J. Huang, et al., Angew. Chem. Int. Ed. 57 (2018) 7804-7808. DOI:10.1002/anie.201803321 |
| [58] |
H.J. Chen, C.Y. Chew, E.H. Chang, et al., J. Am. Chem. Soc. 140 (2018) 5224-5234. DOI:10.1021/jacs.8b01159 |
| [59] |
S. Chen, Y. Hong, Y. Liu, et al., J. Am. Chem. Soc. 135 (2013) 4926-4929. DOI:10.1021/ja400337p |
| [60] |
D. Cheng, J. Peng, Y. Lv, et al., J. Am. Chem. Soc. 141 (2019) 6352-6361. DOI:10.1021/jacs.9b01374 |
| [61] |
M. Collot, T.K. Fam, P. Ashokkumar, et al., J. Am. Chem. Soc. 140 (2018) 5401-5411. DOI:10.1021/jacs.7b12817 |
| [62] |
C.L. Fleming, S. Li, M. Grotli, J. Andreasson, J. Am. Chem. Soc. 140 (2018) 14069-14072. DOI:10.1021/jacs.8b09523 |
| [63] |
J. Ning, T. Liu, P. Dong, et al., J. Am. Chem. Soc. 141 (2019) 1126-1134. DOI:10.1021/jacs.8b12136 |
| [64] |
N.I. Shank, H.H. Pham, A.S. Waggoner, B.A. Armitage, J. Am. Chem. Soc. 135 (2013) 242-251. DOI:10.1021/ja308629w |
| [65] |
X. Wang, P. Li, Q. Ding, et al., J. Am. Chem. Soc. 141 (2019) 2061-2068. DOI:10.1021/jacs.8b11414 |
| [66] |
A.T. Wrobel, T.C. Johnstone, A.D. Liang, S.J. Lippard, P. Rivera-Fuentes, J. Am. Chem. Soc. 136 (2014) 4697-4705. DOI:10.1021/ja500315x |
| [67] |
R. Yan, Y. Hu, F. Liu, et al., J. Am. Chem. Soc. 141 (2019) 10331-10341. DOI:10.1021/jacs.9b03649 |
| [68] |
L. Yuan, W. Lin, Y. Yang, H. Chen, J. Am. Chem. Soc. 134 (2012) 1200-1211. DOI:10.1021/ja209292b |
| [69] |
L. Yuan, W. Lin, S. Zhao, et al., J. Am. Chem. Soc. 134 (2012) 13510-13523. DOI:10.1021/ja305802v |
| [70] |
Y. Wu, S. Huang, J. Wang, et al., Nat. Commun. 9 (2018) 3983. DOI:10.1038/s41467-018-06499-1 |
| [71] |
H.W. Liu, X.X. Hu, K. Li, et al., Chem. Sci. 8 (2017) 7689-7695. DOI:10.1039/C7SC03454G |
| [72] |
Y. Liu, S. Wang, Y. Ma, et al., Adv. Mater. 29 (2017) 1606129. DOI:10.1002/adma.201606129 |
| [73] |
G.K. Park, J.H. Lee, A. Levitz, et al., Adv. Mater. 31 (2019) 1806216. DOI:10.1002/adma.201806216 |
| [74] |
X. Tan, S. Luo, L. Long, et al., Adv. Mater. 29 (2017) 1704196. DOI:10.1002/adma.201704196 |
| [75] |
Y. Wang, S. Luo, C. Zhang, et al., Adv. Mater. 30 (2018) 1800475. DOI:10.1002/adma.201800475 |
| [76] |
H. Li, X. Li, W. Shi, Y. Xu, H. Ma, Angew. Chem. Int. Ed. 57 (2018) 12830-12834. DOI:10.1002/anie.201808400 |
| [77] |
M. Li, A. Lee, K.L. Kim, et al., Angew. Chem. Int. Ed. 57 (2018) 2120-2125. DOI:10.1002/anie.201711629 |
| [78] |
R.R. Nani, A.P. Gorka, T. Nagaya, H. Kobayashi, M.J. Schnermann, Angew. Chem. Int. Ed. 54 (2015) 13635-13638. DOI:10.1002/anie.201507391 |
| [79] |
J. Peng, A. Samanta, X. Zeng, et al., Angew. Chem. Int. Ed. 56 (2017) 4165-4169. DOI:10.1002/anie.201612020 |
| [80] |
D. Su, C.L. Teoh, S.J. Park, et al., Chem 4 (2018) 1128-1138. DOI:10.1016/j.chempr.2018.02.016 |
| [81] |
Z.Q. Xu, X.T. Huang, X. Han, et al., Chem 4 (2018) 1609-1628. DOI:10.1016/j.chempr.2018.04.003 |
| [82] |
W. Sun, S. Guo, C. Hu, J. Fan, X. Peng, Chem. Rev. 116 (2016) 7768-7817. DOI:10.1021/acs.chemrev.6b00001 |
| [83] |
A. Chevalier, Y. Zhang, O.M. Khdour, J.B. Kaye, S.M. Hecht, J. Am. Chem. Soc. 138 (2016) 12009-12012. DOI:10.1021/jacs.6b06229 |
| [84] |
V. Glembockyte, R. Wieneke, K. Gatterdam, et al., J. Am. Chem. Soc. 140 (2018) 11006-11012. DOI:10.1021/jacs.8b04681 |
| [85] |
X. Jia, Q. Chen, Y. Yang, et al., J. Am. Chem. Soc. 138 (2016) 10778-10781. DOI:10.1021/jacs.6b06398 |
| [86] |
N. Karton-Lifshin, L. Albertazzi, M. Bendikov, P.S. Baran, D. Shabat, J. Am. Chem. Soc. 134 (2012) 20412-20420. DOI:10.1021/ja308124q |
| [87] |
Y. Li, Y. Sun, J. Li, et al., J. Am. Chem. Soc. 137 (2015) 6407-6416. DOI:10.1021/jacs.5b04097 |
| [88] |
S.Y. Lim, K.H. Hong, D.I. Kim, H. Kwon, H.J. Kim, J. Am. Chem. Soc. 136 (2014) 7018-7025. DOI:10.1021/ja500962u |
| [89] |
Y. Liu, J. Zhou, L. Wang, et al., J. Am. Chem. Soc. 138 (2016) 12368-12374. DOI:10.1021/jacs.6b04048 |
| [90] |
T. Ma, Y. Hou, J. Zeng, et al., J. Am. Chem. Soc. 140 (2018) 211-218. DOI:10.1021/jacs.7b08900 |
| [91] |
T. Myochin, K. Kiyose, K. Hanaoka, et al., J.Am.Chem.Soc. 133 (2011) 3401-3409. DOI:10.1021/ja1063058 |
| [92] |
J. Yin, Y. Kwon, D. Kim, et al., J. Am. Chem. Soc. 136 (2014) 5351-5358. DOI:10.1021/ja412628z |
| [93] |
K. Zhou, H. Liu, S. Zhang, et al., J. Am. Chem. Soc. 134 (2012) 7803-7811. DOI:10.1021/ja300176w |
| [94] |
X. Zhao, C.X. Yang, L.G. Chen, X.P. Yan, Nat. Commun. 8 (2017) 14998. DOI:10.1038/ncomms14998 |
| [95] |
X. Zheng, X. Wang, H. Mao, et al., Nat. Commun. 6 (2015) 5834. DOI:10.1038/ncomms6834 |
| [96] |
Z. Guo, G.H. Kim, J. Yoon, I. Shin, Nat. Protoc. 9 (2014) 1245-1254. DOI:10.1038/nprot.2014.086 |
| [97] |
J. Yin, Y. Kwon, D. Kim, et al., Nat. Protoc. 10 (2015) 1742-1754. DOI:10.1038/nprot.2015.109 |
| [98] |
Z. Yang, J.H. Lee, H.M. Jeon, et al., J. Am. Chem. Soc. 135 (2013) 11657-11662. DOI:10.1021/ja405372k |
| [99] |
M. Ye, X. Wang, J. Tang, et al., Chem. Sci. 7 (2016) 4958-4965. DOI:10.1039/C6SC00970K |
| [100] |
Z.Q. Guo, Y.G. Ma, Y.J. Liu, et al., Sci. China Chem. 61 (2018) 1293-1300. DOI:10.1007/s11426-018-9240-6 |
| [101] |
N. Kamaly, B. Yameen, J. Wu, O.C. Farokhzad, Chem. Rev. 116 (2016) 2602-2663. DOI:10.1021/acs.chemrev.5b00346 |
| [102] |
X. He, Z. Zhao, L.H. Xiong, et al., J. Am. Chem. Soc. 140 (2018) 6904-6911. DOI:10.1021/jacs.8b02350 |
| [103] |
A. Nicol, R.T.K. Kwok, C. Chen, et al., J. Am. Chem. Soc. 139 (2017) 14792-14799. DOI:10.1021/jacs.7b08710 |
| [104] |
J. Qi, C. Chen, X. Zhang, et al., Nat. Commun. 9 (2018) 1848. DOI:10.1038/s41467-018-04222-8 |
| [105] |
Y. Liu, S. Zhu, K. Gu, et al., ACS Appl. Mater. Inter. 9 (2017) 29496-29504. DOI:10.1021/acsami.7b07091 |
| [106] |
Q. Li, Q. Wang, S. Wang, et al., Adv. Therapeutics 1 (2018) 1800093.
|
| [107] |
Q. Zhou, S. Shao, et al., Nat. Nanotechnol. 14 (2019) 799-809. DOI:10.1038/s41565-019-0485-z |
| [108] |
C. Yan, Z. Guo, Y. Shen, et al., Chem. Sci. 9 (2018) 4959-4969. DOI:10.1039/C8SC01069B |
| [109] |
C. Yan, Z. Guo, Y. Liu, et al., Chem. Sci. 9 (2018) 6176-6182. DOI:10.1039/C8SC02079E |
 2019, Vol. 30
2019, Vol. 30 


