b Analytical & Testing Center, Sichuan University, Chengdu 610064, China;
c College of Light Industry and Textile and Food Engineering, Sichuan University, Chengdu 610065, China
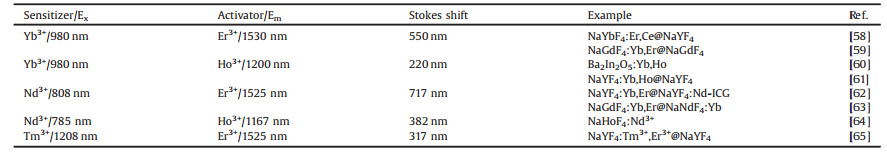
Fluorescence is a dominant technology used extensively in bio-optical sensing owing to the benefits of simple, low-cost and sensitive. One well-known feature of fluorescence is that emission occurs typically at longer wavelength than that of absorption. In order to memory the great contribution of Sir Stokes to the fluorescence theory, such phenomenon was denoted as Stokes shift [1]. Stokes shift is an important characteristic of the fluorophores, which reveals the energy losses between absorption and emission (Scheme 1). Upon absorption, the fluorophore is pumped to the excited states (Sn), followed by rapid relaxation to the first excited state (S1, internal conversion) and then decay back to the ground state (S0, fluorescence). Loss of the excitation energy during internal conversion results in redshifted emission (versus absorption), and therefore Stokes shift (Scheme 1).
|
Download:
|
| Scheme 1. The mechanism of Stokes shift generation from the Jablonski energy diagram of fluorescence. | |
Depending on the Stokes shifts, varied spectral overlap between the absorption and the emission spectra of fluorophores is presented. For the typical organic dyes and fluorescent proteins, usually small Stokes shifts are observed (< 50 nm), resulting in substantial spectral overlap between the absorption and the emission (Scheme 1). Consequently, the fluorescence emission will be absorbed by the fluorophore itself, which is known as selfabsorption. Self-absorption is a typical mechanism of fluorescence quenching that seriously influences the optical performance of the fluorophores [2, 3]. The existence of spectral overlap directly limits the range of excitation wavelength, as it is problematic to excite optimum fluorescence in the overlap range. Besides, self-absorption also causes concentration quenching or aggregation-caused quenching (ACQ) of fluorophores [4]. Therefore, the application of small Stokes shifts-based fluorophores in optosensing is limited due to the relatively small Stokes shift, especially for the sensing ensembles that primarily rely on the spectral overlap, e.g., fluorescence resonance energy transfer- and inner filter effectbased optosensing.
As an important alternative to conventional organic fluorophores, fluorescent nanocrystals possess intriguing features of tunable absorption/emission and better photostability [5-7]. More important, the Stokes shift of some fluorescent nanocrystals can be conveniently tuned, leading to the development of fluorescent materials with minimized or even zero self-absorption [8, 9]. Therefore in this review, the recent progress in exploring large Stokes shift fluorescent nanocrystals for optosensing are summarized, with particular emphasis on eliminating the spectral interferences. Various construction strategies of large Stokes shift-based nanocrystals, including doped quantum dots (QDs), alloy QDs, noble metal nanoclusters and rare earth doped nanocrystals, are discussed. Meanwhile, several advanced applications of large Stokes shift-based nanoprobes used for biological detection and imaging are also introduced.
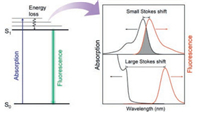
2. Effect of self-absorption on the nanosensorsFluorescent nanocrystal, especially quantum dots (QDs), possess narrow emission, broad excitation and good photostability, but sometimes still suffers from self-absorption [10, 11]. Accordingly, the effective excitation should be narrowed as compared with the original broad excitation, since fluorescence excited by the wavelength in spectral overlap region will be reabsorbed. As a result, the sensitivity of such fluorescence assays is attenuated, especially for inner filter effect (IFE) and fluorescence resonance energy transfer (FRET) based sensing schemes that primarily rely on the spectral overlap (Scheme 2). For a typical IFE sensing system [12], fluorescence modulation relies on absorption of the excitation and/or emission energy of the donor by an absorber. Accordingly, the Stokes shift of the donor should be a critical parameter that determines the assay sensitivity. While for the FRET sensing system based on spectral overlap between energy donor and acceptor [13, 14], not only the Stokes shift of the energy donor, but also the energy acceptor, should be taken into consider.
|
Download:
|
| Scheme 2. Effect of self-absorption on small Stokes shift based nano-biosensing: (A) IFE; (B) FRET; (C) biological assembly. | |
Besides FRET and IFE, the limited brightness of those small Stokes shift-based nanomaterials make it difficult to develop high sensitive fluorescence assays, particularly as fluorescence probes based on fluorescence signal amplification (such as biological assembly, Scheme 2C) [15, 16]. Meanwhile, the existence of selfabsorption also contributes to the decreased photo-stability and quantum yield of these fluorescent nanomaterials. Together with the common autofluorescence interference of biological systems [17], these weaknesses make small Stokes shift-based fluorophores unfavorable for high-quality biological fluorescence labeling, such as fluorescence imaging.
3. Construction of large Stokes shift-based nanocrystalsThe generation of Stokes shift suggests that there is a certain energy dissipation of the excitation before the fluorescence (Scheme 1). Thus, the Stokes shift can be tuned through regulation of the energy loss (the emission wavelength). The emission wavelength of nanocrystals is mainly depended on the band gap, namely the minimum energy required to excite an electron from the valence band to the conduction band [18-21]. Hence, it is possible to enlarge the Stokes shift via regulating the band gap of nanocrystals as well as reduce the spectral overlap between absorption and emission spectra.
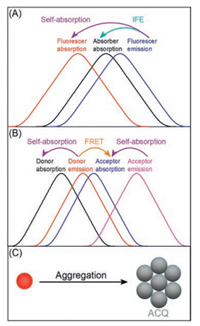
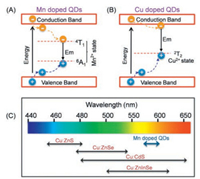
3.1. Doped quantum dotsFluorescence emission of quantum dots (QDs) is the recombination process of the excited state conduction band electrons with the corresponding hole in the valence band, and it is observed that the fluorescence emission can be regulated by adjusting the band gap. Doping intentional impurities (such as Mn2+, Cu2+ and Eu3+) into the lattice of the host QDs is the major way to change the optical, electrical and magnetic properties of QDs, which endow the doped QDs (d-dots) with new characteristics and functions [22-26]. Importantly, with the introduction of doped ions, new d-band energy levels are introduced, providing a new location for electrons or holes to transfer (Fig. 1).
|
Download:
|
| Fig. 1. Exciton relaxation pathways of Mn (A) and Cu (B) doped QDs and their schematic presentation of the range of tunable emission (C). | |
The change of the electron-hole recombination pathways resulting in narrowed emission energy gap and thus red-shifted fluorescence emission of d-dots. Due to the small doping amounts of d-dots, the introduction of transition metal ion dopants causes little influence to the crystal structure and size of the host QDs, leading to almost unchanged absorption as compared with their undoped counterparts. Therefore, d-dots can effectively enlarge the Stokes shift of the original host QDs and avoid the selfquenching problem, leading to popular applications in luminescent solar concentrators [27, 28]. To date, among the various doped QDs, much attention was paid to Mn- and Cu-doped QDs, due to their excellent optical properties and well-developed synthetic knowledge [17, 29].
For Mn2+ doped QDs, as the d-orbital energy levels of Mn2+ are located between the band gap of the most host QDs, and the excited QDs will quickly transfer energy to the d-bands of Mn2+ (Fig. 1A). As a result, the fluorescence of the host QDs is quenched, and the emission wavelength of Mn2+ doped QDs is red shifted to 580 nm (Mn2+ 4T1→ 6A1 transition) [30-33]. As the d-dots still gains energy from the host QDs, the decreased emission energy by Mn2+ doping results in greatly increased Stokes shift of QDs. Moreover, the self-absorption effect can be minimized when Mn2+ ion doped into host QDs with large energy gap, especially ZnS (Eg 3.6 eV) and ZnSe (Eg 2.7 eV), which is appealing for biosensing applications.
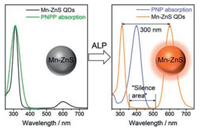
Recently, Mn-doped ZnS QDs were chosen as fluorophore for IFE assay of alkaline phosphatase (ALP, Fig. 2)[34].The absorption of PNPP (p-nitrophenylphosphate, a substrate for alkaline phosphatase, ALP) overlaps considerably with the excitation of Mn-ZnS d-dots, leading to quenched phosphorescence of the Mn-ZnS d-dots through IFE. After ALP enzymatic reaction (PNPP → PNP, p-nitrophenol), the absorption of product PNP no longer overlaps with the excitation of Mn-ZnSd-dots, resulting in phosphorescence turn-on detection of ALP. Importantly, due to the large Stokes shift (up to 300 nm) of Mn-ZnS d-dots, a "silence area" (Fig. 2) was provided for PNP (Absorber) to be located in and eliminates the possible re-absorption of the emission by PNP and itself. Compared with the undoped ZnS QDs with the same excitation as Mn-ZnS d-dots, the emission of undoped ZnS QDs (~450 nm) would be easilyre-absorbed by PNP, thus decreasing the detection sensitivity.
|
Download:
|
| Fig. 2. Mn-doped ZnS d-dots as fluorophore for phosphorescent IFE-based ALP sensing. Copied with permission [34]. Copyright 2017, Royal Society of Chemistry. | |
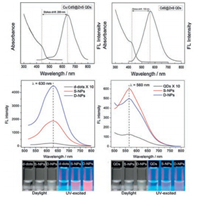
For Cu2+-doped QDs, the T2 state of Cu2+ also locates inside the bandgap of the host QDs. Upon excitation, the hole in the valence band transfers to the T2 state, resulting in dopant emission from the recombination of electron on the conduction band of host QDs and the hole on the Cu T2 state (Fig. 1B). Accordingly, in contrast to the Mn-doped QDs, the emission of Cu-doped QDs is tunable from blue to near-infrared (NIR), depending on the bandgap of the host QDs [35, 36]. In other words, the Stokes shifts of Cu-doped QDs can be tuned through varying the composition and size of the host QDs (Fig. 1C). Harvesting the enlarged Stokes shift of Cu-doped QDs for decreased self-absorption, Li et al. developed a dually-enriched strategy to assemble Cu-doped CdS QDs for generating a highly luminescent fluorescent imaging probe (Fig. 3). No appreciable aggregation-caused quenching was observed from the enriched Cu-doped CdS QDs due to its large Stokes shift (200 nm) that feature almost zero self-absorption. While for undoped CdS QDs assembly, a significant quenching was exist ascribe to its smaller Stokes shift (Fig. 3) [16]. Apparently, large Stokes shift-based nanocrystals are advantageous for highly sensitive bioassays.
|
Download:
|
| Fig. 3. Comparison of the Stokes shift and fluorescence enriched performance between Cu doped QDs and undoped QDs. Copied with permission [16]. Copyright 2015, Royal Society of Chemistry. | |
3.2. Alloy quantum dots
Alloy QDs, including ternary and even quaternary semiconductor QDs, have been intensively investigated due to its unique optical properties. As the band gap of QDs can be regulated by changingtheir composition, it is possible to regulate the band gap of QDs through adjusting the composition of the binary nanocrystals for constructing large Stokes shift based QDs [37-39].Specifically, by changing the ratio of cations oranions in alloyQDs, the bandgap will be regulated so as to change the emission wavelength without changing the size of the QDs [40-42], demonstrating that alloy QDs is one effective way to construct fluorescent nanocrystals with large Stokes shift [43]. For example, the bulk CdS nanocrystals with blue emission color possess a small Stokes shift near 20 nm. After adding Te2- ion to form CdS xTe1-x alloy QDs [44], no significant change was observed in absorption spectrum, while the Stokes shift was greatly enlarged to 270 nm, due to the decreased band gap upon alloying (Fig. 4). Similarly, the Stokes shift of CuxInS alloyed nanocrystals can also be enlarged with the increasing concentration of Cu2+ ion [45]. To date, owning tothe unique optical properties, alloy QDshavebeenwidely used for bioapplications, especially nanocrystals with near-infrared (NIR) emission that was beneficial to eliminate autofluorescence in vivo. However, few sensors were constructed based on the property of large Stokes shift until now. Thus, taking the superiority of large Stokes shift, alloy QDs can be further explored for sensor design with high sensitivity such as enhanced bioimaging.

|
Download:
|
| Fig. 4. The decreasing spectral overlap of CdSxTe1-x (A) and CuxInS (B) alloyed nanocrystals as well as the comparison of band gap and Stokes shift (C). Reproduced with permission [44, 45]. Copyright 2012 and 2008, American Chemical Society. | |
3.3. Fluorescent noble metal nanoclusters
Noble metal nanoclusters (NCs) is a kind of aggregation that typically composed of a few to several hundred metal atoms, including mainly gold naonclusters (AuNCs) [46] and silver nanoclusters (AgNCs) [47]. Compared to semiconductor QDs and organic dyes, noble metal NCs are of special interest as their ultrasmall size (< 2 nm) that close to the fermi level of the electrons, which results in molecule-like properties of discrete electronic states. Furthermore, noble metal NCs with different atomic numbers and ligands show different energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) [48, 49]. In this regard, noble metal NCs can be designed to fluoresce from visible to nearinfrared. For example, upon increasing the molar ratio of Ag/PMAA from 400% to 800%, the absorption peak of poly(methacrylic acid) (PMAA) stabilized silver nanoclusters almost retained, but the center of the emission peak was red-shifted, resulting in increased Stokes shift [50].
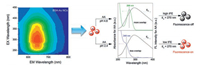
Due to low toxicity and good biocompatibility, noble metal NCs should be a favorable choice as large Stokes shift-based nanoparticles in designing of high sensitive fluorescent assays [51, 52]. Recently, using Cu nanoclusters (NCs) as energy donor, Li et al. developed a FRET-based sensor for GSH. As the emission spectra of Cu NCs did not overlap with the absorption spectrum but exactly overlapped with the absorption spectrum of MnO2 (acceptor), the sensitive and selective detection of GSH in human whole blood samples was successfully realized without selfabsorbing interference [53]. Also in a previous work, BSA protected AuNCs was employed as the fluorophore in IFE system for extreme acidity detection with ascorbic acid as the absorber [54]. The as-prepared NIR-emitting AuNCs possess large Stokes shift near 400 nm, indicating no re-absorption will generate for influencing the detection (Fig. 5). Therefore, on the basis of the regular absorption change of ascorbic acid in extremely acidic pH, the fluorescence of AuNCs showed a good linearity versus pH in the range of 2.4–4.6. The developed probe was also explored for extremely acidic pH detection in several bacteria.
|
Download:
|
| Fig. 5. Large Stokes shift-based AuNCs as fluorophore for IFE-based extreme acidity sensing. Copied with permission [54]. Copyright 2017, Royal Society of Chemistry. | |
3.4. Rare earth doped nanocrystals
Rare earth (Ln) doped nanocrystals is one type of realizable large Stokes shift based fluorescent material due to its tunable energy levels of Ln3+. Especially, Ln-based NIR probes show appealing downshifting luminescence with NaLuF4, NaYF4, CaF2, and LaF3 as host materials [55-57]. Currently, Ho3+- or Er3+-doped nanocrystals have been reported as excellent candidates for deeptissue imaging since they possess emissions at 1200 nm (Ho3+, NIR-II) and 1550 nm (Er3+, NIR-Ⅲ), respectively. Meanwhile, Yb3+ is the most commonly explored co-dopant and sensitizer in Ho3+- or Er3+-doped nanocrystals that possess a relatively strong absorption at 980 nm, realizing large Stokes shift for Ho3+ or Er3+ ions doped nanocrystals (Table 1) [58-65].
|
|
Table 1 Luminescence properties of rare earth doped nanocrystal with different sensitizer and activator. |
To minimize the potential photo-damage to the biological tissue and background absorption in organism, Nd3+ ion is a promising sensitizer due to its relatively large absorption cross-section at 800 nm. Thus, Nd3+ ion is a good replacement of Yb3+ to construct rare-earth doped nanocrystals with enhanced Stokes shift near 750 nm [66-68]. Besides, in order to realize NIR-II wavelength excitation for better imaging resolution, recent report by Zhang has introduced Tm3+ as a new sensitizers that can be excited in the NIR-II window and efficiently transfer energy to Ho3+ and Er3+ [65]. As a consequence, the as-prepared NaYF4:Tm3+, Er3+@NaYF4 nanocrystal can be used to harvest 1208 nm photons from Tm3+and then produce 1550 nm emission by Er3+, which also realizing a large Stokes shift.
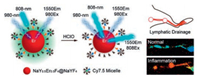
Recently, based on an absorption competition between Cy7.5 fluorophores and Er3+-doped lanthanide nanoparticles at 808 nm, Zhang et al. chose NaErxY1-xF4@NaYF4 nanocrystals as a NIR-II signal unit while Cy7.5 as the competition absorber, developing a 1550 nm emissive ratiometric nanoprobe in response to HOCl with a detection limit down to 500 nmol/L [69]. Due to the reduced light absorption and increased tissue penetration of Er3+-doped nanocrystals, the as-prepared probe further demonstrated its utility for high-resolution ratiometric sensing of lymphatic inflammation in vivo (Fig. 6).
|
Download:
|
| Fig. 6. Schematic illustration showing the ratiometric response of Er3+-doped nanocrystals to HOCl based on an absorption competition-induced emission mechanism. Copied with permission [69]. Copyright 2019, American Chemical Society. | |
4. Conclusions and outlooks
In conclusion, large Stokes shift-based nanocrystals for optical biosensing were summarized in this review. In most fluorescence assays, the detection sensitivity may suffer from the effect of self-absorption, which is a typical mechanism of fluorescence quenching that originates from the overlap between absorption and emission spectra. Consequently, the key to minimizing the self-absorption is to enlarge the Stokes shifts of the fluorophores, which decrease the spectral overlap of absorption and the emission of the fluorophore. Fluorescence nanocrystals with tunable band gap made it possible to enlarge the Stokes shift and resulting in self-absorption reduced.
For constructing large Stokes shift-based nanocrystals, doped QDs is one of the most representative method due to the constant absorption spectrum and increased energy loss upon doping. Mn2+ and Cu2+ doped QDs feature almost zero self-absorption that have widely applied for high sensitivity bioassay and bioimaging. Besides, Mn2+-containing QDs can be used as MRI/fluorescent dual-modal imaging probe [70, 71] while Cu64 doped QDs can be served as PET/ fluorescent dual-modal imaging probe [72]. Thus, combining the property of large stokes shift and multifunctional imaging capability; it is possible to achieve both high sensitivity and high spatial resolution bioimaging. Similarity, alloy QDs can continuously regulate their band gap by adjusting different cation or anion ratios without size change, realizing Stokes shift enlarging. However, the current synthetic protocol for high-quality alloy QDs suitable for aqueous biosensing and bioimaging applications needs further development.
While for noble metal NCs, large Stokes shifted-based nanocrystals can be easily obtained under mild conditions through controlling ligands and other factors, which also show good stability in acidic environment. Yet, most noble metal NCs -based sensors utilize their properties such as small size and low toxic, while sensors that developed based on large Stokes shift have been reported seldom until now. Therefore, the unique of large Stokes shift can be further explored for sensor design.
Among these large Stokes shift-based nanocrystals, rare earth doped nanocrystals have proven to be the best candidate for NIR imaging since the choice of the rare earth dopant allows for the adjustment of both excitation and emission wavelengths. Nevertheless, the disadvantage of low quantum yield for rare earth doped nanocrystals restricts its further application in some cases.
In addition, in order to meet the practical application of various fields, accurately regulating the band gap of these above large Stokes shift-based nanocrystals and endow them specific properties need to be further studied. Besides, the disadvantage of low quantum yield in most large Stokes shift-based nanocrystals has yet to be solved for higher sensitivity. Moreover, for biomedical applications, the potential toxicity of these nanocrystals should be a major concern. Therefore, advanced research is necessary to ensure the safety of large Stokes shift-based nanocrystals for application in biosensing.
AcknowledgmentsThe authors gratefully acknowledge the financial support from the Youth Science Foundation of Sichuan Province (No. 2016JQ0019) and the Postdoctoral Science Foundation of China (No. 2018M633359).
| [1] |
J.R. Lakowicz, Princples of Flourescence Spectroscopy, 3rd ed., Springer, 2006.
|
| [2] |
J. Jung, C.H. Lin, Y.J. Yoon, et al., Angew. Chem. Int. Ed. 55 (2016) 5071-5075. DOI:10.1002/anie.201601198 |
| [3] |
H. Wu, Y. Zhang, M. Lu, et al., J. Nanopart. Res. 18 (2016) 206.
|
| [4] |
Y.N. Hong, J.W.Y. Lam, B.Z. Tang, Chem. Soc. Rev. 40 (2011) 5361-5388. DOI:10.1039/c1cs15113d |
| [5] |
G. Gao, Y.W. Jiang, W. Sun, F.G. Wu, Chin. Chem. Lett. 29 (2018) 1475-1485. DOI:10.1016/j.cclet.2018.07.004 |
| [6] |
D. Yao, Y. Liu, J. Li, H. Zhang, Chin. Chem. Lett. 30 (2019) 277-284. DOI:10.1016/j.cclet.2018.07.012 |
| [7] |
Y.Q. Li, C.Y. Xu, C. Shu, X.D. Hou, P. Wu, Chin. Chem. Lett. 28 (2017) 1961-1964. DOI:10.1016/j.cclet.2017.04.027 |
| [8] |
F. Poulsen, T. Hansen, J. Phys. Chem. C 121 (2017) 13655-13659. DOI:10.1021/acs.jpcc.7b01792 |
| [9] |
S. Mandal, A.C. Reber, M.C. Qian, et al., Acc. Chem. Res. 46 (2013) 2385-2395. DOI:10.1021/ar3002975 |
| [10] |
M. Anni, E. Alemanno, A. Creti, et al., J. Phys. Chem. A 114 (2010) 2086-2090. DOI:10.1021/jp906860c |
| [11] |
Z. Krumer, S.J. Pera, R.J.A. van Dijk-Moes, et al., Sol. Energy Mater. Sol. Cells 111 (2013) 57-65. DOI:10.1016/j.solmat.2012.12.028 |
| [12] |
J.Y. Zhang, R.H. Zhou, D.D. Tang, X.D. Hou, P. Wu, Trends Anal. Chem. 110 (2019) 183-190. DOI:10.1016/j.trac.2018.11.002 |
| [13] |
A.R. Clapp, I.L. Medintz, J.M. Mauro, et al., J. Am. Chem. Soc. 126 (2004) 301-310. DOI:10.1021/ja037088b |
| [14] |
P.R. Selvin, Methods Enzymol. 246 (1995) 300-334. DOI:10.1016/0076-6879(95)46015-2 |
| [15] |
R.H. Zhou, S.K. Sun, C.H. Li, et al., ACS Appl. Mater. Interfaces 10 (2018) 34060-34067. DOI:10.1021/acsami.8b14554 |
| [16] |
M. Li, C.Y. Xu, L. Wu, P. Wu, X.D. Hou, Chem. Commun. 51 (2015) 3552-3555. DOI:10.1039/C4CC10127H |
| [17] |
P. Wu, X.P. Yan, Chem. Soc. Rev. 42 (2013) 5489-5521. DOI:10.1039/c3cs60017c |
| [18] |
D. Lei, Y.T. Shen, Y.Y. Feng, W. Feng, Sci. China-Technol. Sci. 55 (2012) 903-912. DOI:10.1007/s11431-011-4717-1 |
| [19] |
G.Y. Chen, J. Shen, T.Y. Ohulchanskyy, et al., ACS Nano 6 (2012) 8280-8287. DOI:10.1021/nn302972r |
| [20] |
L.H. Gao, F.M. Gao, Appl. Phys. Lett. 103 (2013) 053101. DOI:10.1063/1.4816972 |
| [21] |
Z.Y. He, Z.H. Jin, M. Zhan, et al., Chin. Chem. Lett. 28 (2017) 1851-1856. DOI:10.1016/j.cclet.2017.07.012 |
| [22] |
V. Chikan, J. Phys. Chem. Lett. 2 (2011) 2783-2789. DOI:10.1021/jz2012325 |
| [23] |
D.J. Norris, A.L. Efros, S.C. Erwin, Science 319 (2008) 1776-1779. DOI:10.1126/science.1143802 |
| [24] |
D. Mocatta, G. Cohen, J. Schattner, et al., Science 332 (2011) 77-81. DOI:10.1126/science.1196321 |
| [25] |
M. Makkar, R. Viswanatha, RSC Adv. 8 (2018) 22103-22112. DOI:10.1039/C8RA03530J |
| [26] |
X.L. Ren, M.Q. Wang, X. He, et al., Chin. Chem. Lett. 29 (2018) 1865-1868. DOI:10.1016/j.cclet.2018.12.007 |
| [27] |
C.S. Erickson, L.R. Bradshaw, S. McDowall, et al., ACS Nano 8 (2014) 3461-3467. DOI:10.1021/nn406360w |
| [28] |
M. Sharma, K. Gungor, A. Yeltik, et al., Adv. Mater. 29 (2017) 1700821. DOI:10.1002/adma.201700821 |
| [29] |
N. Pradhan, S. Das Adhikari, A. Nag, D.D. Sarma, Angew. Chem. Int. Ed. 56 (2017) 7038-7054. DOI:10.1002/anie.201611526 |
| [30] |
R. Beaulac, P.I. Archer, S.T. Ochsenbein, D.R. Gamelin, Adv. Funct. Mater. 18 (2008) 3873-3891. DOI:10.1002/adfm.200801016 |
| [31] |
R.H. Zhou, M. Li, S.L. Wang, et al., Nanoscale 6 (2014) 14319-14325. DOI:10.1039/C4NR04473H |
| [32] |
J.Y. Zhang, D.D. Tang, Y.D. Yao, X.D. Hou, P. Wu, Nanoscale 10 (2018) 9236-9244. DOI:10.1039/C8NR02151A |
| [33] |
X.M. Lu, J.Y. Zhang, Y.N. Xie, et al., Anal. Chem. 90 (2018) 2939-2945. DOI:10.1021/acs.analchem.7b05365 |
| [34] |
J.Y. Zhang, X.M. Lu, Y. Lei, X.D. Hou, P. Wu, Nanoscale 9 (2017) 15606-15611. DOI:10.1039/C7NR03673F |
| [35] |
H.B. Shen, H.Z. Wang, X.M. Li, et al., Dalton Trans. (2009) 10534-10540. |
| [36] |
S. Jana, B.B. Srivastava, S. Acharya, et al., Chem. Commun. 46 (2010) 2853-2855. DOI:10.1039/b925980e |
| [37] |
H. Zhong, Z. Bai, B. Zou, J. Phys. Chem. Lett. 3 (2012) 3167-3175. DOI:10.1021/jz301345x |
| [38] |
N.X. Ca, N.T. Hien, N.T. Luyen, et al., J. Alloy Compd. 787 (2019) 823-830. DOI:10.1016/j.jallcom.2019.02.139 |
| [39] |
X. Zhong, M. Han, Z. Dong, T.J. White, W. Knoll, J. Am. Chem. Soc. 125 (2003) 8589-8594. DOI:10.1021/ja035096m |
| [40] |
R.E. Bailey, S.M. Nie, J. Am. Chem. Soc. 125 (2003) 7100-7106. DOI:10.1021/ja035000o |
| [41] |
A.M. Smith, S.M. Nie, J. Am. Chem. Soc. 133 (2011) 24-26. DOI:10.1021/ja108482a |
| [42] |
M.D. Regulacio, M.Y. Han, Acc. Chem. Res. 43 (2010) 621-630. DOI:10.1021/ar900242r |
| [43] |
K. Boldt, N. Kirkwood, G.A. Beane, P. Mulvaney, Chem. Mat. 25 (2013) 4731-4738. DOI:10.1021/cm402645r |
| [44] |
N.P. Gurusinghe, N.N. Hewa-Kasakarage, M. Zamkov, J. Phys. Chem. C 112 (2008) 12795-12800. |
| [45] |
L. de Trizio, M. Prato, A. Genovese, et al., Chem. Mat. 24 (2012) 2400-2406. DOI:10.1021/cm301211e |
| [46] |
L.Y. Chen, C.W. Wang, Z.Q. Yuan, H.T. Chang, Anal. Chem. 87 (2015) 216-229. DOI:10.1021/ac503636j |
| [47] |
J.T. Petty, S.P. Story, J.C. Hsiang, R.M. Dickson, J. Phys. Chem. Lett. 4 (2013) 1148-1155. DOI:10.1021/jz4000142 |
| [48] |
S. Ozkar, R.G. Finke, J. Am. Chem. Soc. 124 (2002) 5796-5810. DOI:10.1021/ja012749v |
| [49] |
C.M. Aikens, Acc. Chem. Res. 51 (2018) 3065-3073. DOI:10.1021/acs.accounts.8b00364 |
| [50] |
I. Diez, M. Pusa, S. Kulmala, et al., Angew. Chem. Int. Ed. 48 (2009) 2122-2125. DOI:10.1002/anie.200806210 |
| [51] |
B. Santiago-Gonzalez, C. Vazquez-Vazquez, M.C. Blanco-Varela, et al., J. Colloid Interface Sci. 455 (2015) 154-162. DOI:10.1016/j.jcis.2015.05.042 |
| [52] |
A.L. Wen, X.X. Peng, P.P. Zhang, et al., Anal.Bioanal.Chem. 410 (2018) 6489-6495. DOI:10.1007/s00216-018-1246-9 |
| [53] |
T.Z. Li, Z.G. Wang, D.F. Jiang, et al., Sens. Actuators B-Chem. 290 (2019) 535-543. DOI:10.1016/j.snb.2019.04.033 |
| [54] |
R.H. Zhou, Y.Q. Li, Y.Y. Tian, et al., Nanoscale 9 (2017) 10167-10172. DOI:10.1039/C7NR03566G |
| [55] |
J.T. Xu, A. Gulzar, P.P. Yang, et al., Coord. Chem. Rev. 381 (2019) 104-134. DOI:10.1016/j.ccr.2018.11.014 |
| [56] |
Y. Qiao, S.H. Li, W.H. Liu, et al., Nanomaterials 8 (2018) 43. DOI:10.3390/nano8010043 |
| [57] |
E. Hemmer, A. Benayas, F. Legare, F. Vetrone, Nanoscale Horiz. 1 (2016) 168-184. DOI:10.1039/C5NH00073D |
| [58] |
X.L. Lei, R.F. Li, D.T. Tu, et al., Chem. Sci. 9 (2018) 4682-4688. DOI:10.1039/C8SC00927A |
| [59] |
Y.F. Wang, G.Y. Liu, L.D. Sun, et al., ACS Nano 7 (2013) 7200-7206. DOI:10.1021/nn402601d |
| [60] |
Z.Y. Wang, H. Jiao, Z.L. Fu, Inorg. Chem. 57 (2018) 8841-8849. DOI:10.1021/acs.inorgchem.8b00739 |
| [61] |
D.J. Naczynski, M.C. Tan, M. Zevon, et al., Nat. Commun. 4 (2013) 2199. DOI:10.1038/ncomms3199 |
| [62] |
W. Shao, G. Chen, A. Kuzmin, et al., J. Am. Chem. Soc. 138 (2016) 16192-16195. DOI:10.1021/jacs.6b08973 |
| [63] |
Y. Fan, P. Wang, Y. Lu, et al., Nat. Nanotechnol. 13 (2018) 941-946. DOI:10.1038/s41565-018-0221-0 |
| [64] |
Y. Feng, Q. Xiao, Y. Zhang, et al., J. Mater. Chem. B 5 (2017) 504-510. DOI:10.1039/C6TB01961G |
| [65] |
H. Zhang, Y. Fan, P. Pei, et al., Angew. Chem. Int. Ed. 58 (2019) 10153-10157. DOI:10.1002/anie.201903536 |
| [66] |
N. Venkatachalam, T. Yamano, E. Hemmer, et al., J. Am. Ceram. Soc. 96 (2013) 2759-2765. DOI:10.1111/jace.12476 |
| [67] |
U. Rocha, C. Jacinto, W.F. Silva, et al., ACS Nano 7 (2013) 1188-1199. DOI:10.1021/nn304373q |
| [68] |
Z.F. Yu, J.P. Shi, J.L. Li, P.H. Li, H.W. Zhang, J. Mater. Chem. B 6 (2018) 1238-1243. DOI:10.1039/C7TB03052E |
| [69] |
S.F. Wang, L. Liu, Y. Fan, et al., Nano Lett. 19 (2019) 2418-2427. DOI:10.1021/acs.nanolett.8b05148 |
| [70] |
Y. Wang, B. Wu, C. Yang, et al., Small 12 (2015) 534-546. |
| [71] |
V.K. Sharma, S. Gokyar, Y. Kelestemur, et al., Small 10 (2014) 4961-4966. DOI:10.1002/smll.201401143 |
| [72] |
X. Sun, X. Huang, J. Guo, et al., J. Am. Chem. Soc. 136 (2014) 1706-1709. DOI:10.1021/ja410438n |
 2019, Vol. 30
2019, Vol. 30