Due to their optical and structural stability, non-invasiveness, and real-time response [1-5], fluorescence imaging as a powerful technology has become an essential tool in a variety of fields, such as clinical diagnostics [6, 7], biotechnology [8], biochemistry, materials science, analytical [9] and environmental chemistry. Classical fluorophores contain coumarin, pyrene, 1, 8-naphthalimide, xanthenes, squaraine, cyanine, boron dipyrromethene difluoride (BODIPY), and nitrobenzofurazan.
As the chromophore developed, BODIPY fluorescent dyes, a structural analogue of the porphyrins, have been attracting the attention of researchers over the last three decades. Since the BODIPY dyes were first discovered in 1968 [10] (Fig. 1), their use as photoelectric materials have also been explored [11]. Subsequently, they have been extensively applied in biotechnology as fluorescent markers or for small-molecule detection. BODIPY dyes have also been used in medicine for imaging living cells and animals in preclinical research. BODIPY has been considered a potential scaffold because of their neutral total charge, sharp absorption, and emission with high fluorescence quantum yield. In addition, they are scarcely sensitive to the polarity and pH of their environment and are extremely stable to physiological conditions [12, 13]. However, traditional BODIPY chromophore has two major drawbacks that are the less than 600 nm emission wavelength and it is hydrophobicity [14-16].
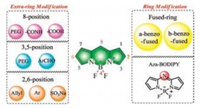
|
Download:
|
| Fig. 1. The modification of BODIPY. | |
Near-infrared (NIR, 650–900 nm) light could not only penetrate further deeply into tissues without significant damage to biological samples but also prevent the interference from background autofluorescence of biological samples in the living systems [17-20]. In addition, good solubility in water is also beneficial for its application in biological systems.
Interestingly, the properties of BODIPY, such as absorption/emission wavelength and hydrophilicity, are easily altered by even minor modifications to the structures of BODIPY [21, 22]. Red-shifts of the absorption and emission wavelength have been achieved by conjugating group substitution at the 2/6-, 3/5-position, aromatic ring fusion with the pyrrole moiety and aza-nitrogen atom substitution at the meso-position to form an aza-BODIPY [23-25]. The focus of this review is to summarize the modification of BODIPY including improving the emission wavelength and water-solubility in order to extend the application.
2. Peripheral substitution of BODIPY for water-soluble and NIR 2.1. 8/meso-Substituted BODIPYThe modification at the 8/meso-position has little effect on the absorption and emission wavelengths, so researchers tend to pay more attention to improve the water solubility of molecules via introducing a neutral group such as a water-soluble polymer chain 1-4, a carboxyl anion 5, 6 or an amphiphilic ion 7-9. The synthesis of these compounds would be relatively simpler and the conditions would not be strict by this method. The water-soluble modifying group has certain stability in the organism, which has potential application value for cell imaging (Fig. 2).
|
Download:
|
| Fig. 2. Selected structurally-modified water-soluble BODIPYs 1-9. | |
In nature, branched oligo(ethylene glycol) methyl ether plays an important role in ensuring water solubility. As shown in Fig. 3, the water-soluble BODIPYs 1 and 2 conjugated with poly(ethylene glycol) at the 8/meso-position were designed for responding to biological thiols and NO via photo-induced electron transfer (PET) and intramolecular charge transfer (ICT) mechanisms [26-28].

|
Download:
|
| Fig. 3. (A) PEG-based BODIPY probe 1. (B) Fluorescent probe 2 based on BODIPY. | |
Another design strategy for improving water solubility was developed by Lingling Li and other groups. This type of watersoluble BODIPYs usually contains the acid-functional substituent such as carboxylic acid. These materials 5, 6 indicate highly fluorescence in aqueous environments and effective labeling through the plasma membrane [29, 30] (Fig. 2).
It is also worth highlighting a nature-inspired molecular modification strategy to evaluate biocompatibility of BODIPYs by introducing amidothiourea group. For example, BODIPY-amide dye 7 achieves a low detection to F- from sodium fluoride solution (Fig. 2), via test-strip-based fluorometric detection [31]. The Gustavo Fernandez group used layered self-assembled BODIPYamidothiourea dyes to enhance the substantial antitumor activity of capsaicin in vivo in prostate cancer. The target 8 displayed an obvious emission changes while cell uptake-induced disassembly, which successfully reduced the dose of CAP drugs in prostate cancer [32] (Fig. 2).
Recently, by integrating post-polymerization modification with thiosemicarbazide, the fluorescent probe 9 is able to selective detect and quantitative separate Hg(Ⅱ) ions of toxic ions presenting in aqueous media (Fig. 4). The probe displayed bright turn-on fluorescent emission when exposed to Hg(Ⅱ) ions [33].
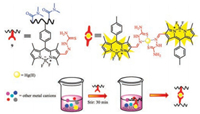
|
Download:
|
| Fig. 4. Schematic representation of the Hg2+ removal process by 9. | |
2.2. 2/6-Substituted BODIPY
Summarizing the literature on the modification of the fluoroboron dipyrrole at 2/6-position, of modifying water solubility and the near-infrared field has been discovered. At the 2/6- position, the hydrophilic group such as a sulfonate or a carboxylate is often added to increasing the water solubility, at the same time, the conjugated system of the molecule results in the increase of fluorescence emission via π-π conjugated system (Fig. 5).
|
Download:
|
| Fig. 5. Chemical structure of water-soluble and NIR probes 10-19. | |
However, in contrast to 3/5-position, the red shift value of the 2/6-position is not satisfied. Generally, the conjugated emission wavelength is concentrated between 600 nm and 700 nm, so in recent years, more groups introducing in the 3/5 site to achieve a larger emission wavelength, a higher fluorescence quantum yield, and a larger Stokes shift.
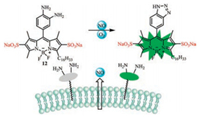
In early years, disodium sulfate has been introduced into small organic molecules as a common water-soluble group. Watersoluble fluorescence probes 10 and 11 with disodium sulfates at 2, 6-position were synthesized to study biocatalytic Diels-Alder reactions (Fig. 5), using the aromatic system [34]. In Fig. 6, Hong Wang's group reported an amphiphilic fluorescent probe for the release of visual detection of NO outside living cells. Probe 12 contains disodium 2, 6-disulfonate moiety to remain the fluorophore and recognition extracellularly and a hydrophobic C16 alkyl chain acts as the membrane anchor [35].
|
Download:
|
| Fig. 6. Structure of 12 and possible recognition mechanism for NO. | |
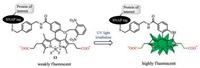
Among various water-soluble groups, carboxylates are attracting more and more attention from researchers due to their ease of preparation and stability [36]. Yasuteru Urano designed a new class of caged BODIPY 13 fluorophores for spatiotemporal imaging of living cell epidermal growth factor receptor (EGFR) and SNAP tag fusion proteins (Fig. 7).
|
Download:
|
| Fig. 7. Illustration of probe 13 when UV light irradiation. | |
The near-infrared fluorescent molecules have good tissue penetration and strong anti-interference ability, to meet these objectives, fluorescent dyes should possess double bond conjugated with BODIPY to expand resonance system. A series of 2, 6-p-dimethylaminostyrene isomers 14 and 15 were developed as sensors for changes in pH (Fig. 5), which showed markedly redshifted absorbance bands, very low fluorescence quantum yields and significant Stokes shifts [37]. Recently, researchers reported a series of 2-alkenyl- and 2, 6-dialkenylboron dipyrromethene (BODIPY) derivatives 16 and 17 using Pd(Ⅱ)-catalyzed regioselective and stereoselective oxidative C--H olefination as reaction mechanism. This probe has great potential value in bioimaging (Fig. 8) [38].

|
Download:
|
| Fig. 8. Synthesis of probes 16 and 17. | |
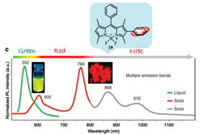
Conjugation of the BODIPY directly at the 2, 6-position through the aromatic ring is also a method to make the fluorescent molecule near-infrared. Selenophene-substituted BODIPY 18 [39] exhibits good absorption/emission properties in the range of NIR to red (Fig. 5). Moreover, some of the selenophene-substituted BODIPYs have good photosensitizers to generate singlet oxygen. Later, Ling Huang and Wei Huang reported a series of 2-/2, 6-aryl substituted BODIPY dyes. As shown in Fig. 9, the dye 19 exhibits a wide range of red and near-infrared and multi-fluorescence emission in its aggregated state [40].
|
Download:
|
| Fig. 9. Structural detail of 19 and normalized photoluminescence (PL) spectra of 19 in solution (green curve) and microcrystalline powder state (red and gray curves). Copied with permission [40]. Copyright 2018, Springer Nature. | |
2.3. 3/5-Substituted BODIPY
The modification at the 3/5-position is mostly used to increase the emission wavelength of the fluorescent dyes, so it is nearinfrared. The water-soluble peptide and long chain of polyethylene glycol to obtain a probe molecule which is both near-infrared and water-soluble; the modification of the near-infrared dyes is mostly based on the conjugated benzene ring, and the connection between the single and the double is alternated to large conjugated structure (Fig. 10).
|
Download:
|
| Fig. 10. Chemical structures of probes 20-35. | |
The crucial role of poly(ethylene glycol) (PEG) chain in organic molecules is to improve water solubility. Gang Han and co-workers reported a water-soluble carbazole-substituted BODIPY 20 (CarBDP) molecule by introducing PEG chain (Fig. 11). The probe provides biocompatibility and efficient near-infrared (NIR)-light absorption for photodynamic therapy (PDT) in vivo [41]. Following this work, several other PEG-based BODIPYs 21-23 have been synthesized to apply in the endoplasmic reticulum of cells or visualize metastatic breast cancer including bone and liver micrometastases (Fig. 10). They can be used for monitoring HNO levels or detecting micrometastases and specific targeting the bone metastasis [42, 43]. He's group used aryl-based BODIPY polymeric vesicles 24 for photodynamic therapy (PDT) under 660 nm irradiation and photothermal therapy (PTT) under 785 nm irradiation [44] (Fig. 10).
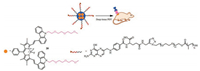
|
Download:
|
| Fig. 11. The application of probe 20 for PDT. | |
It is well known that the 3, 5-methyl BODIPY substituents are sufficiently acidic to react with the aromatic aldehyde (Fig. 12). That has been identified as one of the best strategies for obtaining BODIPY derivatives in the red/NIR region, which are useful for applications such as labeling reagents, photodynamic therapy, chemical sensors and laser dyes.
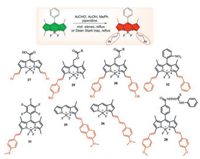
|
Download:
|
| Fig. 12. The condensation reaction of 3, 5-methyl-BODIPY with aromatic aldehydes and structure of probes 25-32. | |
Jong Seung Kim and colleagues developed probes 25 and 26, which have excellent photophysical properties in both solution and cell-based experiments and high selectivity for the selfassembled microtubule-associated protein tau (Tau protein) [45]. Probe 26 uses BODIPY and known Aβ plaque binding dyes for conjugation and extension of cyclization (Fig. 13).
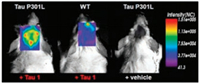
|
Download:
|
| Fig. 13. Biological living experiment of probe 26. Copied with permission [45]. Copyright 2017, American Chemical Society. | |
In 2016, Petr Klán's group designed and synthesized CORBODIPY-based fluorescent probes 27 for detecting transition-metal free carbon monoxide-releasing molecules (Fig. 12). The fluorescence intensity exhibited ratiometric signal at visible-to-NIR (up to 730 nm) light in the presence of CO [46].
Subsequently, various groups design and synthesize a series of near-infrared fluorescent dyes based on this method. The Yi-ping Cui's group synthesized a two-photon NIR ClO- probe 28 with aryl modification for naked-eye detection and mitochondrial localization (Fig. 12). The probe displayed obvious color change, low detection limit and successful mitochondrial imaging via thiosemicarbazide desulfurization reaction [47].
Arthur H. Winter and co-workers reported a class of BODIPYderived photocages 29 and 30 with appended styryl groups achieving long-wavelength absorption (Fig. 12), shorter excitedstate lifetimes and diminished excited-state energies by a direct single-photon-release mechanism. This work gives a possibility that target and control releasement of pharmaceuticals or other biomolecules by red light in the biological window [48].
Another probe 31 that designed upon oxidation of the thioether group into sulfoxide showed high brightness red emission and a noticeable ratiometric fluorescence response towards the exogenous and endogenous HClO/ClO- with high signal-to-noise ratios in living HeLa cells, zebrafish, and mice [49] (Fig. 12).
Recently, A hypoxic monitoring of cardiomyocyte-specific and nitroreductase-activated near-infrared nanoprobes 32 was designed to help estimate the degree of ischemia and guide individualized treatment (Fig. 14). This probe 32 contains a peptide encapsulating nitrobenzene substituted BODIPY (GGGGDRVYIHPF) which can be used for nitroreductase imaging. This work will promote the investigation of the physiological and pathological processes of hypoxic-ischemic heart disease [15].
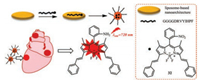
|
Download:
|
| Fig. 14. Schematic illustration of structure and function of probe 32 for NTRactivatable imaging of ischemia-induced myocardial hypoxia. | |
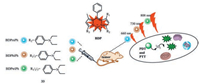
As described previously, three phenyl-based BODIPY compounds 33 were developed (Fig. 15). These three compounds (BDPmPh, BDPbiPh and BDPtriPh) could be easily controlled to achieve an adjustable penetration depth and a low pH maintained in the lysosome. That could improve the efficacy of PDT and PTT [50].
|
Download:
|
| Fig. 15. Illustration of the preparation and application of the BDPmPh, BDPbiPh and BDPtriPh NPs. | |
As shown in Fig. 10, Victor N. Nemykin developed new NIR absorbing platforms processing ferrocene–BODIPY merocyanine dyads 34 and 35, which are susceptible to protonation [51]. If these systems are applied in light capture modules, they could simultaneously target the relaxation dynamic components of fast (primarily charge transfer) and slow (mainly centered on the p system).
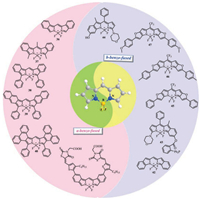
3. Fused-ring BODIPYsTo some extent, the free rotation of the aryl substituent at the 3, 5-position limits the red shift of the main fluorescent band. Various strategies have been employed to achieve greater degree of uniformity between the p-systems of aryl substituents by forming a rigid fused ring system with sp3 hybridized carbon. In the fused ring BODIPY system, the absorption maximum has a significant red shift (Fig. 16).
|
Download:
|
| Fig. 16. Structures of fluorescent fused-probes 36-47. | |
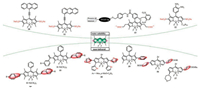
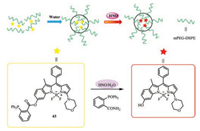
Intense demand for efficient photovaltaic material, organic solar cells and biological applications motivated researchers to synthesize NIR absorbing dyes. Among the strategies to transfer the absorption of BODIPYs to longer wavelength, it is particularly promising to carry out π-extension by fusing an aromatic unit with an a- or b-bond of a pyrrole polymer. A class of benzo-fused and furan fused BODIPYs were synthesized for NIR bioimaging via palladium(Ⅱ)-catalyzed and Paal Knorr pyrrole synthesis or monitoring nitroxyl in some aspects and vacuum processable organic solar cells [45, 52-55]. These probes all exhibits marked red-shift in the absorption and emission and high (photo)chemical stability due to π-π accumulation. Remarkably, the probe 43 (PBODIPY-N) (Fig. 17) contains an amphiphilic copolymer (mPEGDSPE) enveloping P-BODIPY-N into the hydrophobic interior for recognizing nitroxyl [56].
|
Download:
|
| Fig. 17. Molecule structure of probe 43 and mechanism of micellar nanoprobe (NPBODIPY-N) for recognition of HNO. | |
4. Aza-BODIPY dyes
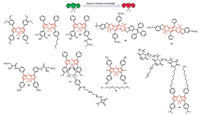
Aza-BODIPY has been a class of heteroatom-containing BODIPY analogues which is beneficial due to the near infrared region, low autofluorescence of biomolecules, less scattering background, and the applicability of low-cost excitation sources (bright red-light emitting diodes (LEDs)) and photodetectors (silicon photodiodes). It is worth noting that aza-BODIPY has excellent light stability, making it an ideal fluorophore for long-term measurement (Fig. 18).
|
Download:
|
| Fig. 18. Chemical structures of fluorogenic probes 44-52. | |
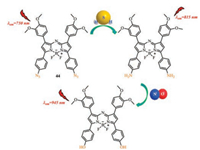
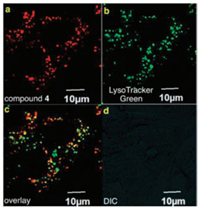
Many kinds of novel NIR absorbing aza-BODIPY derivatives were designed due to their good characteristics [57-60]. The synthesized aza-BODIPYs 44 can apply to interact with hydrogen sulfide (H2S), nitrite ions (NO2-) and nitric oxide (NO) in aqueous medium respectively (Fig. 19), image in 4T1 murine breast cancer cells 50 (Fig. 20) and highly sensitive ammonia sensors for environmental monitoring 47 and 48 (Fig. 18). The aza-BODIPY dyes usually show a profound red-shift remarkable operational stability, low limit of detection (LOD) and the high detectable concentration was obtained in substitution from azido to amino to dimethyl amino groups.
|
Download:
|
| Fig. 19. The structure of probe 44 and plausible mode of interaction between probe 44 and H2S, NO. | |
|
Download:
|
| Fig. 20. (a) Probe 50 was internalized in murine 4T1 breast cancer cells. (b) Colocalized with the intracellular fluorescence. (c) and (d) Bright field image for reference. Copied with permission [60]. Copyright 2015, Royal Society of Chemistry. | |
Derong Cao's group created a new way to synthesize novel nearinfrared pyrrolopyrrole aza-BODIPY luminogens facilely through aggregation-enhanced emission characteristic. The two triphenylethylene-modified pyrrolopyrrole aza-BODIPY dyes featured great dispersity in water and biocompatibility.
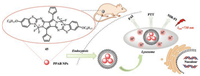
Simultaneously, Dong and co-workers utilized pyrrolopyrrole aza-BODIPY (PPAB) NPs 45 as a photothermal agent for photoacoustic imaging (PAI) and NIR fluorescence imaging (NIR-FI) guided PTT in vivo [61]. Furthermore, the photothermal ablation of tumor cells can be achieved in vitro and in vivo, and PPAB NPs do not have any significant toxicity in mice even at low concentrations (0.5 mg/kg) and in vivo PTT (Fig. 21).
|
Download:
|
| Fig. 21. Schematic illustration of PPAB NPs 45 for NIR-FI/PAI imaging guided PTT. | |
Impressively, a new family of water-soluble and biocompatible aza-BODIPY fluorophores 46 and 51 have been developed [62-64]. Probes possess dipyrromethene or intelligent polymer–MnO2 nanoparticles for in vivo optical imaging studies or dual-activated photoacoustic and magnetic resonance bimodal imaging of live mice, which is highly water-soluble, very stable in physiological media, free in PBS, high in quantum yield, and finally, they can be easily conjugated to antibody organisms (Fig. 18).
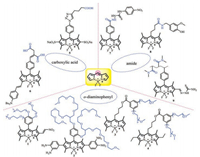
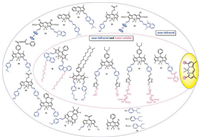
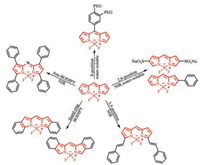
5. Conclusions and outlookIn this review, we summarized BODIPY-based water-soluble and near-infrared molecules that can be applied in vivo imaging, photodynamic therapy etc., modified at different positions. A significant red shift in the maximum of UV absorption and fluorescence emission of BODIPY have been achieved by aryl, ethynyl and styryl substituted at 2/6, 3/5, the fused aromatic ring BODIPY and the aza nitrogen atom is substituted for the meso-carbon atom to form a nitrogen heteroatom. Up to now, many water-soluble BODIPY derivatives have been prepared by introducing oligo(ethylene glycol) chains, nucleotides, sulfonated peptides, carboxylates, sulfonates, phosphonates or betaine moieties (Fig. 22). The progress in preparing p-extension dyes that luminesce in the 650–680 nm window is very limited. In most cases, solubility is achieved by attaching a polar group such as a negatively charged sulfonate to the dye. However, a negative charge can be problematic because it could hinder the binding of negatively charged biological analytes.
|
Download:
|
| Fig. 22. The strategies of designing water-soluble and NIR BODIPY dyes. | |
It is expected that the future development of BODIPYs will be applied more on biological living and photoelectric materials, which demand better hydrophilicity and nearinfrared wavelength. Chemists and biologists need to work in collaboration to explore easier ways. We would hence anticipate a rapid research and development in water-soluble and nearinfrared BODIPYs.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21572177, 21673173 and 21807087), China Postdoctoral Science Foundation (No. 2017M623225), Key Research and Development Program of Shaanxi (No. 2019KWZ-07), the Key Science and Technology Innovation Team of Shaanxi Province (No. 2017KCT-37), Natural Science Basic Research Plan in Shaanxi Province of China (No. 2018JQ3038), and the Xi'an City Science and Technology Project (No. 2017085CG/RC048 (XBDX004)).
| [1] |
X. Chen, F. Wang, J.Y. Hyun, et al., Chem. Soc. Rev. 45 (2016) 2976-3016. DOI:10.1039/C6CS00192K |
| [2] |
J.L. Kolanowski, F. Liu, E.J. New, Chem. Soc. Rev. 47 (2018) 195-208. DOI:10.1039/C7CS00528H |
| [3] |
Y. Xia, R. Zhang, Z. Wang, J. Tian, X. Chen, Chem. Soc. Rev. 46 (2017) 2824-2843. DOI:10.1039/C6CS00675B |
| [4] |
D. Wu, A.C. Sedgwick, T. Gunnlaugsson, et al., Chem. Soc. Rev. 46 (2017) 7105-7123. DOI:10.1039/C7CS00240H |
| [5] |
M. Gao, F.B. Yu, C.J. Lv, J. Choo, L.X. Chen, Chem. Soc. Rev. 46 (2017) 2237-2271. DOI:10.1039/C6CS00908E |
| [6] |
Y. Chen, L. Li, W. Chen, H. Chen, J. Yin, Chin. Chem. Lett. 30 (2019) 1353-1360. DOI:10.1016/j.cclet.2019.02.003 |
| [7] |
Y. Zhu, W. Lin, W. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1875-1877. DOI:10.1016/j.cclet.2017.06.017 |
| [8] |
D. Yue, M. Wang, F. Deng, et al., Chin. Chem. Lett. 29 (2018) 648-656. DOI:10.1016/j.cclet.2018.01.046 |
| [9] |
W. Qin, C. Xu, Y. Zhao, et al., Chin. Chem. Lett. 29 (2018) 1451-1455. DOI:10.1016/j.cclet.2018.04.007 |
| [10] |
A. von.Treibs, F.H. Kreuzer, Liebigs Ann. Chem. 718 (1968) 208-223. DOI:10.1002/jlac.19687180119 |
| [11] |
R.E. Hermes, T.H. Allik, S. Chandra, J.A. Hutchinson, Appl. Phys. Lett. 63 (1993) 877-879. DOI:10.1063/1.109887 |
| [12] |
N. Boens, V. Leen, W. Dehaen, Chem. Soc. Rev. 41 (2012) 1130-1172. DOI:10.1039/C1CS15132K |
| [13] |
S. Kolemen, E.U. Akkaya, Coord. Chem. Rev. 354 (2018) 121-134. DOI:10.1016/j.ccr.2017.06.021 |
| [14] |
T. Kowada, H. Maeda, K. Kikuchi, Chem. Soc. Rev. 44 (2015) 4953-4972. DOI:10.1039/C5CS00030K |
| [15] |
Y. Fan, M. Lu, X.A. Yu, et al., Anal. Chem. 91 (2019) 6585-6592. |
| [16] |
H. He, Coord. Chem. Rev. 273-274 (2014) 87-99. DOI:10.1016/j.ccr.2013.11.006 |
| [17] |
K.D. Piatkevich, F.V. Subach, V.V. Verkhusha, Chem. Soc. Rev. 42 (2013) 3441-3452. DOI:10.1039/c3cs35458j |
| [18] |
K.G. Chernov, T.A. Redchuk, E.S. Omelina, V.V. Verkhushaa, Chem. Rev. 117 (2017) 6423-6446. DOI:10.1021/acs.chemrev.6b00700 |
| [19] |
Z. Guo, S. Park, J. Yoon, I. Shin, Chem. Soc. Rev. 43 (2014) 16-29. DOI:10.1039/C3CS60271K |
| [20] |
H. Xiang, J. Cheng, X. Ma, X. Zhou, J.J. Chruma, Chem. Soc. Rev. 42 (2013) 6128-6185. DOI:10.1039/c3cs60029g |
| [21] |
A. Loudet, K. Burgess, Chem. Rev. 107 (2007) 4891-4932. DOI:10.1021/cr078381n |
| [22] |
J.Z. Zhao, K.J. Xu, W.B. Yang, Z.J. Wang, F.F. Zhong, Chem. Soc. Rev. 44 (2015) 8904-8939. DOI:10.1039/C5CS00364D |
| [23] |
H. Lu, J. Mack, Y. Yang, Z. Shen, Chem. Soc. Rev. 43 (2014) 4778-4823. DOI:10.1039/C4CS00030G |
| [24] |
A. Kamkaew, S.H. Lim, H.B. Lee, et al., Chem. Soc. Rev. 42 (2013) 77-88. DOI:10.1039/C2CS35216H |
| [25] |
A. Turksoy, D. Yildiz, E.U. Akkaya, Coord. Chem. Rev. 379 (2019) 47-64. DOI:10.1016/j.ccr.2017.09.029 |
| [26] |
M. Isik, T. Ozdemir, I.S. Turan, S. Kolemen, E.U. Akkaya, Org. Lett. 15 (2013) 216-219. DOI:10.1021/ol303306s |
| [27] |
G.K. Vegesna, S.R. Sripathi, J. Zhang, et al., ACS Appl. Mater. Interfaces 5 (2013) 4107-4112. DOI:10.1021/am303247s |
| [28] |
I.W. Badon, J. Lee, T. Pegarro Vales, B.K. Cho, H.J. Kim, J. Photochem. Photobiol. A:Chem. 377 (2019) 214-219. DOI:10.1016/j.jphotochem.2019.03.050 |
| [29] |
L. Li, J. Han, B. Nguyen, K. Burgess, J. Org. Chem. 73 (2008) 1963-1970. DOI:10.1021/jo702463f |
| [30] |
K.M. Bardon, S. Selfridge, D.S. Adams, et al., ACS Omega 3 (2018) 13195-13199. DOI:10.1021/acsomega.8b01487 |
| [31] |
P. Ashokkumar, H. Weisshoff, W. Kraus, K. Rurack, Angew. Chem. Int. Ed. 53 (2014) 2225-2229. DOI:10.1002/anie.201307848 |
| [32] |
A. Sampedro, A. Ramos-Torres, C. Schwoppe, et al., Angew. Chem. Int. Ed. 57 (2018) 17235-17239. DOI:10.1002/anie.201804783 |
| [33] |
U. Haldar, H.I. Lee, ACS Appl. Mater. Interfaces 11 (2019) 13685-13693. DOI:10.1021/acsami.9b00408 |
| [34] |
A. Nierth, A.Yu. Kobitski, G.U. Nienhaus, A. Jaschke, J. Am. Chem. Soc. 132 (2010) 2646-2654. DOI:10.1021/ja9084397 |
| [35] |
H.W. Yao, X.Y. Zhu, X.F. Guo, H. Wang, Anal. Chem. 88 (2016) 9014-9021. DOI:10.1021/acs.analchem.6b01532 |
| [36] |
T. Kobayashi, T. Komatsu, M. Kamiya, et al., J. Am. Chem. Soc. 134 (2012) 11153-11160. DOI:10.1021/ja212125w |
| [37] |
L. Gai, J. Mack, H. Lu, et al., Chemistry 20 (2014) 1091-1102. DOI:10.1002/chem.201303291 |
| [38] |
J. Wang, Y. Li, Q. Gong, et al., J. Org. Chem. 84 (2019) 5078-5090. DOI:10.1021/acs.joc.9b00020 |
| [39] |
D. Taguchi, T. Nakamura, H. Horiuchi, M. Saikawa, T. Nabeshima, J. Org. Chem. 83 (2018) 5331-5337. DOI:10.1021/acs.joc.8b00782 |
| [40] |
D. Tian, F. Qi, H. Ma, et al., Nat. Commun. 9 (2018) 2688. DOI:10.1038/s41467-018-05040-8 |
| [41] |
L. Huang, Z. Li, Y. Zhao, et al., J. Am. Chem. Soc. 138 (2016) 14586-14591. DOI:10.1021/jacs.6b05390 |
| [42] |
F. Ali, S. Sreedharan, A.H. Ashoka, et al., Anal. Chem. 89 (2017) 12087-12093. DOI:10.1021/acs.analchem.7b02567 |
| [43] |
H. Xiong, H. Zuo, Y. Yan, et al., Adv. Mater. 29 (2017) 1700131. DOI:10.1002/adma.201700131 |
| [44] |
H. He, S. Ji, Y. He, et al., Adv. Mater. 29 (2017) 1606690. DOI:10.1002/adma.201606690 |
| [45] |
P. Verwilst, H. Kim, J. Seo, et al., J. Am. Chem. Soc. 139 (2017) 13393-13403. DOI:10.1021/jacs.7b05878 |
| [46] |
E. Palao, T. Slanina, L. Muchova, et al., J. Am. Chem. Soc. 138 (2016) 126-133. DOI:10.1021/jacs.5b10800 |
| [47] |
B. Shen, Y. Qian, Z. Qi, et al., J. Mater. Chem. B Mater. Biol. Med. 5 (2017) 5854-5861. DOI:10.1039/C7TB01344B |
| [48] |
J.A. Peterson, C. Wijesooriya, E.J. Gehrmann, et al., J. Am. Chem. Soc. 140 (2018) 7343-7346. DOI:10.1021/jacs.8b04040 |
| [49] |
C. Duan, M. Won, P. Verwilst, et al., Anal. Chem. 91 (2019) 4172-4178. DOI:10.1021/acs.analchem.9b00224 |
| [50] |
J. Zou, P. Wang, Y. Wang, et al., Chem. Sci. 10 (2019) 268-276. DOI:10.1039/C8SC02443J |
| [51] |
Y.V. Zatsikha, N.O. Didukh, D. Nemez, et al., Chem. Commun. (Camb.) 53 (2017) 7612-7615. DOI:10.1039/C7CC03332J |
| [52] |
S. Yamazawa, M. Nakashima, Y. Suda, R. Nishiyabu, Y. Kubo, J. Org. Chem. 81 (2016) 1310-1315. DOI:10.1021/acs.joc.5b02720 |
| [53] |
N. Zhao, S. Xuan, Z. Zhou, et al., J. Org. Chem. 82 (2017) 9744-9750. DOI:10.1021/acs.joc.7b01940 |
| [54] |
O. Suryani, Y. Higashino, J.Y. Mulyana, et al., Chem. Commun. (Camb.) 53 (2017) 6784-6787. DOI:10.1039/C7CC02730C |
| [55] |
L. Jean-Gerard, W. Vasseur, F. Scherninski, B. Andrioletti, Chem. Commun. (Camb.) 54 (2018) 12914-12929. DOI:10.1039/C8CC06403B |
| [56] |
S. Yuan, F. Wang, G. Yang, et al., Anal. Chem. 90 (2018) 3914-3919. DOI:10.1021/acs.analchem.7b04787 |
| [57] |
N. Adarsh, M.S. Krishnan, D. Ramaiah, Anal. Chem. 86 (2014) 9335-9342. DOI:10.1021/ac502849d |
| [58] |
M. Strobl, A. Walcher, T. Mayr, I. Klimant, S.M. Borisov, Anal. Chem. 89 (2017) 2859-2865. DOI:10.1021/acs.analchem.6b04045 |
| [59] |
L. Li, L. Wang, H. Tang, D. Cao, Chem. Commun. (Camb.) 53 (2017) 8352-8355. DOI:10.1039/C7CC04568A |
| [60] |
A.B. Kamkaew, K. Burgess, Chem. Commun. (Camb.) 51 (2015) 10664-10667. DOI:10.1039/C5CC03649F |
| [61] |
C. Wu, X. Huang, Y. Tang, et al., Chem. Commun. (Camb.) 55 (2019) 790-793. DOI:10.1039/C8CC07768A |
| [62] |
J. Pliquett, A. Dubois, C. Racoeur, et al., Bioconjug. Chem. 30 (2019) 1061-1066. DOI:10.1021/acs.bioconjchem.8b00795 |
| [63] |
X. Miao, W. Hu, T. He, et al., Chem. Sci. 10 (2019) 3096-3102. DOI:10.1039/C8SC04840A |
| [64] |
X. Hu, C. Zhan, Y. Tang, et al., Chem. Commun. (Camb.) 55 (2019) 6006-6009. DOI:10.1039/C9CC02148E |
 2019, Vol. 30
2019, Vol. 30 









