b Key Laboratory of Radiopharmaceuticals, Ministry of Education, College of Chemistry, Beijing Normal University, Beijing 100875, China
Biothiols, including cysteine (Cys), homocysteine (Hcy), and glutathione (GSH), are involved in a number of physiological processes and play important roles in keeping the appropriate redox status in complex biological environments [1]. A lot of research has demonstrated that abnormal level of Cys/Hcy/GSH is closely associated with a variety of diseases [2, 3]. Among them, Cys, as the source of sulfide in iron-sulfur clusters, is the precursor of GSH and acetyl coenzyme A [4, 5]. Cys deficiency is associated with many human diseases such as skin lesions, neurotoxicity, lethargy, edema, muscle weakness, slow growth, and hair depigmentation. Elevated Hcy levels in plasma is a risk factor for cardiovascular diseases, Alzheimer's, folate and cobalamin (vitamin B12) deficiency. Moreover, plasma total homocysteine concentrations are also concerned with birth defects, cognitive impairment in the elderly. GSH, as the most abundant intracellular nonprotein thiol, is an endogenous antioxidant and plays the critical roles in the maintenance of intracellular redox activities, metabolism, and intracellular signal transduction. Meanwhile, GSH concentration level is directly related to many diseases, such as cancer, cardiovascular and Alzheimer's diseases. Therefore, the effective methods for detecting differentially GSH/Cys/Hcy are urgently needed in view of important roles in the biosystems.
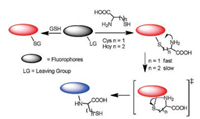
Among the detection methods, optical sensing using fluorescent probes has attracted a great deal of attention in basis of their operational simplicity, relative low cost, noninvasive, high sensitivity and selectivity. Up to date, the design strategies of fluorescent probes for biothiols [6-12] have been involved in various mechanisms, such as Michael addition, cyclization reaction, conjugate addition-cyclization, cleavage of sulfonamide and sulfonate ester, cleavage of disulphide and Se-N bond. These strategies have been summarized in previously reported reviews [13-15]. However, they are unable to discriminate among thiolcontaining molecules in view of their similarity in structure and reactivity. The cyclization of Cys/Hcy with aldehydes or acrylates provided the effective tactics for selective detection of Cys/Hcy over GSH [16, 17]. However, effective means to identify GSH over Cys/Hcy still face significant challenges due to lack of a versatile strategy.Our group pioneeringly proposed an "aromatic nucleophilic substitution-rearrangement (SNAr-rearrangement)" mechanism (Fig. 1), which provided a powerful tool to design fluorescent probes for the discrimination among biothiols. The reaction mechanism is that a labile leaving group of a fluorophore is replaced by thiolates of biothiols through thiol-halogen nucleophilic substitution and the amino groups of Cys/Hcy but not GSH further replace the thiolate to form amino-substituted products. Therefore, the difference of emission of sulfur- and amino-substituted dyes enabled the recognition of GSH over Cys/Hcy. In this review we will focus on the current progress of fluorescent probes for biothiols and some other analytes based on "SNAr-rearrangement" mechanism and inquire into the tendency of development in future.
|
Download:
|
| Fig. 1. The mechanism of reaction between probe and thiol with "SNArrearrangement". Reproduced by permission [15]. Copyright 2015, The Royal Society of Chemistry. | |
2. Fluorescent probes for the selective detection among thiols based on "SNAr-rearrangement" using different fluorophores
A suitable fluorophore is the key element for constructing such a selective probe. It should (1) carry a reactive electrophilic site to enable its response to biothiols and (2) manifest photophyscial properties sufficiently distinct from those of the products of its reaction with the biothiols. The design of fluorescent probes based on fluorophores that met the two criteria was exploited for the selective detection of biothiols using "SNAr-rearrangement" mechanism, such as difluoroboron dipyrromethene (BODIPY), nitrobenzoxadiazole (NBD), cyanine, pyronin, naphthalimide, coumarin [18, 19].
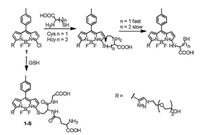
2.1. BODIPYOur group developed a series of BODIPY-based fluorescent probes 1-5 for the discrimination among biothiols. Probe 1 was a chlorinated BODIPY that showed yellow fluorescence with emission band centered at 556 nm (Fig. 2) [20]. In the presence of GSH, thioether gradually formed with emission maximum at 588 nm. The ratio of fluorescence intensities at the emission maxima of the generated thioether (1-S) and the emission ratio of probe 1 (I588nm/I556nm) was proportional to the GSH concentration (0–60 μmol/L), allowing the ratiometric sensing of GSH. Probe 1 was used to imaging intracellular GSH in HeLa cells.
|
Download:
|
| Fig. 2. Reactions of 1 with Cys, Hcy and GSH. | |
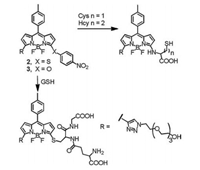
Probe 1 is not suitable for the detection of Cys due to the relatively weaker fluorescence of amines compared to chlorinated BODIPY. In order to design a fluorescent probe for Cys, we replaced the chlorine of probe 1 with nitrophenol or nitrothiophenol moieties, and the resultant probes 2 and 3 showed almost no fluorescence due to the photoinduced electron transfer (PET) (Fig. 3) [21]. The fluorescence intensity was obviously recovered with the nucleophilic substitution of thiol, and the fast intramolecular rearrangement reaction in the presence of Cys rapidly formed N-substituted product so as to distinguish Cys from Hcy and GSH with the spectrum property difference.
|
Download:
|
| Fig. 3. Reactions of probe 2/3 with Cys, Hcy and GSH. | |
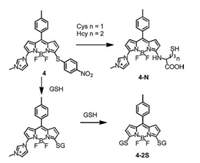
However, probes 1 and 2 showed slow reaction kinetics especially the rearrangement reaction for Hcy. In addition, the difference among the fluorescence maxima of Cl-, S- and Nsubsitituted BODIPY was less than 30 nm. To address these limitations, we regulated the reaction rate and degree between probe and biothiols by inducing different substituent at 3-position of BODIPY. Thus, 3, 5-dichlorinated BODIPY was substituted with imidazolium group and nitrothiophenol to develop probe 4 [22]. The imidazolium group accelerated the substitution rearrangement of Cys and Hcy with extremely fast kinetics to yield 4-N. The rearrangement reaction for Hcy is complete within 2 min. By contrast, probe 1 would take 2 h to go through this process. In the case of GSH, imidazolium group was further substituted by excess GSH so that spectrum of 4-2S was clearly distinct from that of 4-N with great differences in absorption and emission spectra of 120 nm and 68 nm, respectively (Fig. 4). Probe 4 was applied to simultaneously detect GSH and Cys in living cells from different emission channels.
|
Download:
|
| Fig. 4. Reactions of probe 4 toward Cys/Hcy, and GSH. | |
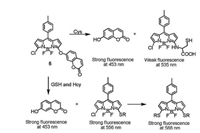
Moreover, we synthesized probe 5 containing coumarin fluorophore that acted as both leaving group and internal standard [23]. Upon addition of Cys, it yielded nitrogen-substituted BODIPY with weak fluorescence and free coumarin with blue emission. In the presence of GSH, it generated mono- and di-sulfur substituted BODIPY and coumarin, resulting in various emission colors at different concentrations of GSH (Fig. 5).
|
Download:
|
| Fig. 5. Probe 5 for distinguishing Cys and GSH. | |
Probes 6-9 (Figs. 6 and 7) were also developed by our group based on the similar mechanism. The excess probe 6 reacted with Cys, Hcy and GSH to yield different main products with distinct polarities (Fig. 6). After simple separation by TLC, simultaneous determination of Cys, Hcy and GSH was achieved. Probe 6 was successfully applied for the simultaneous quantitative detection of Cys/Hcy/GSH in human serum, and the results were in good agreement with those obtained via high performance liquid chromatography (HPLC) [24]. We further displaced the F atoms of 6 by cyano groups to synthesize probe 7 in order to increase the reactivity. The different reactivity of 6 and 7 in the presence of a mixture of biothiols led to distinct absorption spectra at a specified period of time. Together with spectral analysis, we are capable of quantitatively detecting Cys, Hcy and GSH in a mixture [25]. Probe 8 consisted of hydrophobic BODIPY unit and hydrophilic PEG chains, which co-assembled with fluorescein to give non-fluorescent nanoparticles due to the aggregation caused quenching (ACQ). After reacting with GSH, the highly polar GSHsubstituted BODIPY showed high solubility in water, resulting in the disassembly of the nanoparticles to release the highly fluorescent BODIPY derivative (λem = 595 nm) and fluorescein (λem = 512 nm), enabling the dual emissive detection of GSH. A dual-channel responsive fluorescent probe enables fluorescence variations at two different emission channels, which provides a built-in correction for environmental effects and allows more reliable detection. This nanoprobe was applied for the dualchannel fluorescence imaging of GSH in living HeLa cells [26]. Mitochondria are an important subcellular organelle, which is pivotal place to provide ATP for cells and involved in the event of ROS-induced apoptosis. Therefore, it is important to explore GSH level in mitochondria. We integrated triphenylphosphonium into the chlorinated BODIPY to form the probe 9, which can selectively detect mitochondrial GSH (Fig. 7) [27]. Furthermore, the Zhao group presented fluorescent probes 10 and 11, which can simultaneously detect Cys, Hcy, and GSH in view of three distinct fluorescence turn-on responses (Fig. 7) [28]. The Ahn group developed probe 12, in which the methylthio group in meso-position of BODIPY was exploited as leaving group for the selective detection Cys/Hcy over GSH (Fig. 7) [29].

|
Download:
|
| Fig. 6. Probe 6 for simultaneous detection of Cys, Hcy and GSH with polarity difference. | |

|
Download:
|
| Fig. 7. The structures of probes 7-12. | |
2.2. NBD
NBD derivatives with highly electronwithdrawing nitro moiety enable rapid "SNAr-rearrangement" upon addition of Cys/Hcy to yield amino-substituted dyes with strong yellow fluorescence with emission maximum at 546 nm. On the contrary, NBD reacts with GSH generated almost non-fluorescent thioether. Therefore, NBDbased fluorescent probes are suitable for the detection of Cys/Hcy over GSH. We initially used a commercially available chromophore 4-chloro-7-nitro-2, 1, 3-benzoxadiazole (NBD-Cl, probe 13) as fluorescent probes for selective detection of Cys/Hcy over GSH (Fig. 8) [30]. NBD-F (probe 14) [31] and NBD-SCN (probe 15) [32] were also employed as fluorescent probe for differential detection of biothiols. Yin et al. developed the aryl-thioether substituted NBD derivatives 16 as a pH-dependent fluorescent probe to detect biothiols. Probe 16 showed enhancement in the presence of Cys and Hcy in pH 7.4; by contrast, only Cys promoted emission enhancement in pH 6.0 (Fig. 8) [33].

|
Download:
|
| Fig. 8. Reactions of probes 13–16 with biothiols. | |
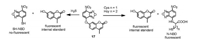
In the design of NBD-based probes, a second fluorophore with phenolic hydroxy group was usually linked with NBD, such as coumarin, resorufin and semi-heptamethine cyanine, which was served as an internal standard [34-39]. This strategy expanded the capability of the probes for the detection of H2S and GSH. For example, Pluth group constructed the nitrobenzofurazan-coumarin probe 17 (Fig. 9) [40]. Cys/Hcy reacted with 17 to generate N-NBD with fluorescence at 549 nm, whereas H2S produced nonfluorescent SH-NBD. Both Cys/Hcy and H2S release free coumarin emitting at 449 nm as an internal standard, allowing the ratiometric measurement of N-NBD versus SH-NBD, and thus the estimation of the concentrations of Cys/Hcy or H2S. Probe 17 was applied to differentiate and report on different oxidative stress stimuli in simulated sulfur pools containing H2S, Cys, and Hcy.
|
Download:
|
| Fig. 9. Probe 17 for the detection of H2S and Cys/Hcy. | |
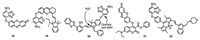
Ma's group developed the probe 18 combining resorufin with NBD to detect Cys (Fig. 10). With the single excitation wavelength of 470 nm, Cys produced two emissions at 540 nm and 585 nm while GSH generated only one emission at 585 nm. Therefore, Cys can be selectively detected from GSH with intramolecular rearrangement mechanism. Probe 18 was demonstrated to detect Cys and GSH in human plasmas [41]. The Zeng group combined the NBD moiety with semi-heptamethine cyanine to form the probe 19 (Fig. 10), which exhibited a significant fluorescence "turn-on" signal towards GSH at 702 nm. In contrast, with addition of Cys, the probe not only displayed the emission at 702 nm but also showed another emission band at 540 nm. Therefore, this probe can simultaneously detect Cys and GSH by different emission channels. Probe 19 was used for quantitative determination of Cys and GSH in human plasmas and in living cells [42]. Moreover, the Lin group connected the hydroxyphenyl benzothiazole merocyanine (HBTMC) fluorophore and NBD to form the probe 20 (Fig. 10). HBTMC moiety had a recognition site for the selective response to H2S, and the NBD moiety was designed for the selective detection of Cys/Hcy and the sequential sensing of Cys/Hcy/GSH and H2S. Probe HMN was applied to discriminate biothiols in living cells by three-color fluorescence imaging [43]. The dual-fluorophore fragmentation strategy was also employed for the construction of fluorescent probes 21 and 22 (Fig. 10) [44, 45].
|
Download:
|
| Fig. 10. Structures of probes 18-22. | |
2.3. Cyanine
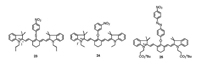
We employed commercially available heptamethine cyanine (IR-780) as selective fluorescent probe for Cys [30]. Cyanine, as a NIR dye, has advantages in bioapplication because of deep tissue penetration, minimum photodamage to biological samples, and minimuminterference fromauto-fluorescence of biomolecules in the living systems. The amino-substituted cyanine emits fluorescence centered at ~750 nm, whereas sulfur-substituted cyanine shows emission at ~820 nm, which was used for the selective detection of Cys/Hcy and/or GSH. The fluorescence of probe 23 (Fig. 11) was quenched by nitrothiophenol group with PET. The fluorescence was turned on when nitrothiophenol was replaced by thiol, and the more rapid intramolecular displacement of sulfur with the amino group of Cys than Hcy/GSH enabled the selective detection Cys over Hcy and GSH [46]. Tang's group [47] synthesized a series of heptamethine cyanine dyes with different substituents in the central position and screened probe 24 (Fig. 11) that showed simultaneous fluorescence sensing ability towards GSH and Cys under single excitation. The heptamethine-azo dye conjugate 25 (Fig. 11) was synthesized by Kim et al. to selectively detect mitochondrial GSH over Cys/Hcy, which introduced the tunable lipophilic cation unit as the biomarker for mitochondria and a nitroazo group as the GSH recognition reaction site and the fluorescence quencher [48]. The fluorescence intensity at 810 nm was enhanced when the nitroazo was substituted with sulfydryl of GSH and weak fluorescence appeared in the presence of Cys/Hcy with "SNArrearrangement" toward probe. Probe 25 was used to selectively detect mitochondrial GSH in cells. However, the rearrangement reaction between cyanine and Hcy is slow, which means it usually takes long time for the discrimination between Hcy and GSH.
|
Download:
|
| Fig. 11. Structures of probes 23-25. | |
2.4. Pyronin
Aminopyronin dyes derived from primary amines absorb at ca. 460 nm and emit at ca. 540 nm; however, thiopyronin dye absorbs and emits at significantly longer wavelength (λabs = 598 nm; λem = 619 nm); moreover, both of them possess high extinction coefficients and quantum yields. The distinct photophysical properties of aminopyronin and thiopyronin dyes make them have potential in the simultaneous detection of Cys/Hcy and GSH. It is difficult to further derive on pyronin dyes, thus limited fluorescent probes for thiols based on pyronin were reported. Guo's group designed 4-methoxythiophenol-substituted pyronin dye as fluorescent probe 26 (Fig. 12), which can differentiate GSH from Cys and Hcy. The Cys/Hcy executed substitution-rearrangement reaction and GSH induced only substitution reaction toward the probe, which led to the corresponding aminopyronin and thiopyronin dyes with distinct photophysical properties so that Cys/Hcy and GSH can be monitored via visible and NIR emission channels, respectively. Probe 26 was applied for simultaneous detection of Cys/Hcy and GSH in B16 cells [49].

|
Download:
|
| Fig. 12. Probe 26 for the differentiation of GSH from Cys and Hcy. | |
2.5. Naphthalimide
Amino-naphthalimide showed bright yellow emission, which could be used for the detection of Cys. Qian's group reported the 4- nitro-1, 8-naphthalic anhydride as fluorescent probe 27 for the detection of Cys. Probe 27 is colorless and non-fluorescent. In the presence of Cys, probe 27 changed from colorless to green with enhanced yellow fluorescence at 530 nm. However, the detection was carried out at 50 ℃ in DMF because of the low reactivity, which made it difficult for bioapplication. (Fig. 13) [50]. In order to improve the reaction kinetics and water solubility, electronwithdrawing and hydrophilic heterocycles were introduced to form probe 28. Under physiological conditions, probe 28 turned into green-emitting amino analogs and blue-emitting thioether in the presence of Cys and GSH, respectively, allowing the discrimination between Cys and GSH (Fig. 13) [51].
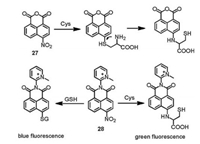
|
Download:
|
| Fig. 13. Probes 27 and 28 to detect biothiols. | |
2.6. Coumarin
Coumarin fluorophores are usually integrated with other reaction sites to construct multiple-binding-site probes that not only enable excellent discrimination among Cys, Hcy and GSH but also allow for their simultaneous detection and accurate ratiometric monitor [52-55]. The substituted coumarin as fluorescent probe 29 was designed with highly selective and extremely rapid detection of GSH over Cys/Hcy (Fig. 14) [56]. GSH substituted the phenyl-selenium group and underwent intramolecular cyclization with aldehyde through forming an iminium-group. However, the Cys/Hcy underwent cyclization with aldehyde to form thiazolidine and substitution reaction and S, N-displacement at the 4-position of coumarin, which resulted the distinct difference of photophysical properties compared with that of GSH. Moreover, Guo's group reported a chlorinated coumarin-hemicyanine probe 30 (Fig. 15) with three potential reaction sites, which can simultaneously sense Cys and GSH with different reaction forms [57]. The Cys-induced substitution-rearrangement-cyclization, Hcy-induced substitution-rearrangement, and GSH-induced substitution-cyclizatioin cascade reaction formed the corresponding aminocoumarin, amino-coumarin-hemicyanine, thiol-coumarin with distinct difference of photophysical properties, therefore the probe can selectively detect Cys and GSH by different emission channels. Moreover, Yin's group designed the probe 31/32 (Fig. 15) with four reaction sites can simultaneously distinguish the Cys, Hcy and GSH, and probe 32 was successfullyapplied tovisualize endogenousHcy/ Cys/GSH and their transformation in living cells [58, 59]. More examples about multiple binding site probes with discrimination of Cys, Hcy and GSH were highlighted and summarized in the recently published review literatures [60, 61].
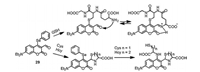
|
Download:
|
| Fig. 14. The mechanism of probe 29 to detect thiols. | |
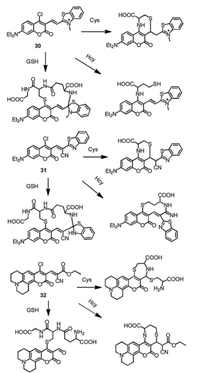
|
Download:
|
| Fig. 15. The Multiple reaction site probes 30-32 and their reactions with thiols. | |
3. Fluorescent probes based on "SNAr-rearrangement" for the detection of other analytes
The "SNAr-rearrangement" mechanism was not only used for the selective detection of thiols, but also for other analytes. Probe 33 was designed by the Zhao group to detect γ-glutamyltranspeptidase (GGT) (Fig. 16) [62-64]. Probe 33 was synthesized by linking GSH to BODIPY fluorophore to yield sulfur-substituted BODIPY. In presence of GGT, the cleavage of γ-glutamyl linkage would liberate free amino group, followed by an intramolecular rearrangement of thiolate to yield an amino-substituted BODIPY, which showed a hypochromic shift of ~20 nm accompanied by a remarkable fluorescence enhancement, so that GGT can be detected. Guo's group reported a mitochondria-specific fluorescent probe 34 for dual-channel detection of nitric oxide (NO) assisted by intracellular Cys and GSH (Fig. 16). Probe 34 was constructed by directly connecting the pyronin dye with the one amino group of o-phenylenediamino (OPD, a classic NO detection group), which is non-fluorescent due to the PET mechanism. OPD reacted with NO to form benzotriazole derivatives 34-T, resulted in fluorescence recover. Moreover, in the presence of intracellular Cys and GSH, 34-T further transformed into a green emission aminopyronin 34-N and a red-emission thiopyronin 34-S, respectively, enabling the monitor of mitochondrial NO [65]. The same group also reported a dual-channel fluorescence probe 35 for diagnosing a wide range of cancer cell types assisted by organic-anion transporting polypeptide (OATP) transporters and Cys/GSH. It displays the capability of dual-fluorescence diagnosis of cancer tissues from tumour xenograft models of mice and harvested surgical specimens of patients [66]. Recently, we reported probe 36 by introducing 4-amino-3-(methylamino)-phenol to BODIPY, which can simultaneously detect NO and GSH. The NO-mediated transformation of diamine into a triazole triggered the fluorescence off-on response in the green channel, whereas the GSHinduced SNAr substitution reaction led to the red-shifted emission in the red channel [67]. Probe 36 was demonstrated to visualize the elevation of both NO and GSH in inflammatory mediator.
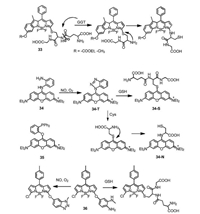
|
Download:
|
| Fig. 16. The mechanism of the reaction of probes 33-36 with other analytes. | |
4. Conclusions
This review summarized the design of fluorescent probes based on "SNAr-rearrangement" mechanism for selective discrimination among Cys, Hcy and GSH. A fluorophore carrying a labile substituent reacts with thiols to yield a thioether by SNAr. The amino group of Cys/Hcy (but not GSH) further displaces sulfur through a 5- or 6-membered ring transition state to generate amino products. Moreover, the selective discrimination of GSH, Cys, and/or Hcy relies on the distinct photophysical properties of thioether- and amino-substituted dyes. A suitable fluorophore plays key roles for construction of desirable probes. We classified these probes by the types of fluorophores, including BODIPY, nitrobenzofurazan, cyanine, pyronin, naphthalimide and coumarin. This mechanism provides a creative new direction in the field of selective discrimination among Cys, Hcy and GSH, and much progress has been made on simultaneous, accurate ratiometric monitoring of biothiols. However, there are still significant challenges in the field of thiols detection. Hcy is the one-carbon homologue of Cys. The selective detection of Hcy is rarely reported, and mainly relies on the slower reaction kinetics of intramolecular rearrangement compared to Cys, which still suffers from the interference from Cys or GSH. In addition, the total concentration of Hcy in plasma is low, which makes the selective detection even harder. On the other hand, the simultaneous detection of biothiols in real biosamples is still a challenge, because when biothiols coexist in the mixture the probes might suffer from the interference from each other, and other competing species in the complex biological environment. Furthermore, in biological applications, most of the probes were used to image biothiols in living cells, or detect biothiols in human plasma. More details about the concentration and distribution of thiols and even their transformations related to physiological and pathological processes should be investigated more deeply, both in vitro and in vivo. Overall, we hope this review will encourage continued efforts in the development of new fluorescent probes for biothiols and other relevant analytes, and further study their functions in living systems.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (No. 21525206).
| [1] |
T.V. Mishanina, M. Libiad, R. Banerjee, Nat. Chem. Biol. 11 (2015) 457-464. DOI:10.1038/nchembio.1834 |
| [2] |
B. Debreceni, L. Debreceni, Cardiovasc. Ther. 32 (2014) 130-138. DOI:10.1111/1755-5922.12064 |
| [3] |
C. Harris, J.M. Hansen, Methods Mol. Biol. 889 (2012) 325-346. |
| [4] |
B.D. Paul, S.H. Snyder, Nat. Rev. Mol. Cell Biol. 13 (2012) 499-507. DOI:10.1038/nrm3391 |
| [5] |
B.D. Paul, J.I. Sbodio, S.H. Snyder, Trends Pharmacol. Sci. 39 (2018) 513-524. DOI:10.1016/j.tips.2018.02.007 |
| [6] |
Z. Xu, X. Huang, X. Han, et al., Chem 4 (2018) 1609-1628. DOI:10.1016/j.chempr.2018.04.003 |
| [7] |
K. Umezawa, M. Yoshida, M. Kamiya1, et al., Nat. Chem. 9 (2017) 279-286. DOI:10.1038/nchem.2648 |
| [8] |
Y. Yue, F. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181-3185. DOI:10.1021/jacs.6b12845 |
| [9] |
M. She, Z. Wang, T. Luo, et al., Chem. Sci. 9 (2018) 8065-8070. DOI:10.1039/C8SC03421D |
| [10] |
H. Chen, Y. Tang, M. Ren, et al., Chem. Sci. 7 (2016) 1896-1903. DOI:10.1039/C5SC03591K |
| [11] |
H. Nie, L. Qiao, W. Yang, et al., J. Mater. Chem. B 4 (2016) 4826-4831. DOI:10.1039/C6TB00938G |
| [12] |
L. Song, X.D. Sun, Y. Ge, et al., Chin. Chem. Lett. 27 (2016) 1776-1780. DOI:10.1016/j.cclet.2016.05.007 |
| [13] |
X. Chen, Y. Zhou, X. Peng, et al., Chem. Soc. Rev. 39 (2010) 2120-2135. DOI:10.1039/b925092a |
| [14] |
H.S. Jung, X. Chen, J.S. Kim, et al., Chem. Soc. Rev. 42 (2013) 6019-6031. DOI:10.1039/c3cs60024f |
| [15] |
L.Y. Niu, Y.Z. Chen, H.R. Zheng, et al., Chem. Soc. Rev. 44 (2015) 6143-6160. DOI:10.1039/C5CS00152H |
| [16] |
O. Rusin, N.N. St. Luce, R.A. Agbaria, et al., J. Am. Chem. Soc. 126 (2004) 438-439. DOI:10.1021/ja036297t |
| [17] |
X. Yang, Y. Guo, R.M. Strongin, Angew. Chem. Int. Ed. 50 (2011) 10690-10693. DOI:10.1002/anie.201103759 |
| [18] |
Q. Liu, C. Zhang, X. Wang, et al., Chem.-Asian J. 11 (2016) 202-206. DOI:10.1002/asia.201501013 |
| [19] |
H. Zhang, R. Liu, J. Liu, et al., Chem. Sci. 7 (2016) 256-260. DOI:10.1039/C5SC02431E |
| [20] |
L.Y. Niu, Y.S. Guan, Y.Z. Chen, et al., J. Am. Chem. Soc. 134 (2012) 18928-18931. DOI:10.1021/ja309079f |
| [21] |
L.Y. Niu, Y.S. Guan, Y.Z. Chen, et al., Chem. Commun. 49 (2013) 1294-1296. DOI:10.1039/c2cc38429a |
| [22] |
L.Y. Niu, Q.Q. Yang, H.R. Zheng, et al., RSC Adv. 5 (2015) 3959-3964. DOI:10.1039/C4RA13526A |
| [23] |
X.L. Liu, L.Y. Niu, Y.Z. Chen, et al., Biosens. Bioelectron. 90 (2017) 403-409. DOI:10.1016/j.bios.2016.06.076 |
| [24] |
M.Y. Jia, L.Y. Niu, Y. Zhang, et al., ACS Appl. Mater. Interfaces 7 (2015) 5907-5914. DOI:10.1021/acsami.5b00122 |
| [25] |
L.Y. Niu, M.Y. Jia, P.Z. Chen, et al., RSC Adv. 5 (2015) 13042-13045. DOI:10.1039/C4RA16601A |
| [26] |
Y. Liu, L.Y. Niu, Y.Z. Chen, et al., J. Photochem. Photobiol. A 355 (2018) 311-317. DOI:10.1016/j.jphotochem.2017.08.042 |
| [27] |
X.L. Liu, L.Y. Niu, Y.Z. Chen, et al., Org. Biomol. Chem. 15 (2017) 1072-1075. DOI:10.1039/C6OB02407F |
| [28] |
F. Wang, Z. Guo, X. Li, et al., Chem.-Eur. J. 20 (2014) 11471-11478. DOI:10.1002/chem.201403450 |
| [29] |
D.H. Ma, D. Kim, E. Seo, et al., Analyst 140 (2015) 422-427. DOI:10.1039/C4AN01791A |
| [30] |
L.Y. Niu, H.R. Zheng, Y.Z. Chen, et al., Analyst 139 (2014) 1389-1395. DOI:10.1039/c3an01849k |
| [31] |
L. Song, L.M. Ma, Q. Sun, et al., Chin. Chem. Lett. 27 (2016) 330-334. DOI:10.1016/j.cclet.2015.12.012 |
| [32] |
Y.H. Chen, J.C. Tsai, T.H. Cheng, et al., Biosens. Bioelectron. 56 (2014) 117-123. DOI:10.1016/j.bios.2014.01.009 |
| [33] |
D. Lee, G. Kim, J. Yin, et al., Chem. Commun. 51 (2015) 6518-6520. DOI:10.1039/C5CC01071C |
| [34] |
Q. Hu, C. Yu, X. Xia, et al., Biosens. Bioelectron. 81 (2016) 341-348. DOI:10.1016/j.bios.2016.03.011 |
| [35] |
X. Yang, L. He, K. Xu, et al., Anal. Chem. Acta 981 (2017) 86-93. DOI:10.1016/j.aca.2017.05.016 |
| [36] |
P. Wang, Y. Wang, N. Li, et al., Sens. Actuators B-Chem. 245 (2017) 297-304. DOI:10.1016/j.snb.2017.01.127 |
| [37] |
X. Qiu, X. Jiao, C. Liu, et al., Dye. Pigments 140 (2017) 212-221. DOI:10.1016/j.dyepig.2017.01.047 |
| [38] |
Z. Ye, C. Duan, Q. Hu, et al., J. Mater. Chem. B 5 (2017) 3600-3606. DOI:10.1039/C7TB00489C |
| [39] |
J. Zhang, X. Ji, J. Zhou, et al., Sens. Actuators B-Chem. 257 (2018) 1076-1082. DOI:10.1016/j.snb.2017.10.133 |
| [40] |
M.D. Hammers, M.D. Pluth, Anal. Chem. 86 (2014) 7135-7140. DOI:10.1021/ac501680d |
| [41] |
X. Gao, X. Li, L. Li, et al., Chem. Commun. 51 (2015) 9388-9390. DOI:10.1039/C5CC02788H |
| [42] |
J. Xu, J. Pan, Y. Zhang, et al., Sens. Actuators B-Chem. 238 (2017) 58-65. DOI:10.1016/j.snb.2016.07.038 |
| [43] |
L. He, X. Yang, K. Xu, et al., Chem. Sci. 8 (2017) 6257-6265. DOI:10.1039/C7SC00423K |
| [44] |
H. Zhang, L. Xu, W. Chen, et al., Anal. Chem. 91 (2019) 1904-1911. DOI:10.1021/acs.analchem.8b03869 |
| [45] |
J. Pan, Y. Zhang, J. Xu, et al., RSC Adv. 5 (2015) 97781-97787. DOI:10.1039/C5RA21025A |
| [46] |
Y.S. Guan, L.Y. Niu, Y.Z. Chen, et al., RSC Adv. 4 (2014) 8360-8364. DOI:10.1039/c3ra47116k |
| [47] |
X. Wang, J. Lv, X. Yao, et al., Chem. Commun. 50 (2014) 15439-15442. DOI:10.1039/C4CC06637E |
| [48] |
S.Y. Lim, K.H. Hong, D.I. Kim, et al., J. Am. Chem. Soc. 136 (2014) 7018-7025. DOI:10.1021/ja500962u |
| [49] |
J. Liu, Y.Q. Sun, H. Zhang, et al., Chem. Sci. 5 (2014) 3183-3188. DOI:10.1039/c4sc00838c |
| [50] |
L. Ma, J. Qian, H. Tian, et al., Analyst 137 (2012) 5046-5050. DOI:10.1039/c2an35624d |
| [51] |
L. Song, T. Jia, W. Lu, et al., Org. Biomol. Chem. 12 (2014) 8422-8427. DOI:10.1039/C4OB01219D |
| [52] |
K. Xiong, F. Huo, J. Chao, et al., Anal. Chem. 91 (2019) 1472-1478. DOI:10.1021/acs.analchem.8b04485 |
| [53] |
Y. Lia, W. Liu, P. Zhang, et al., Biosens. Bioelectron. 90 (2017) 117-124. DOI:10.1016/j.bios.2016.11.021 |
| [54] |
S.V. Mulay, Y. Kim, M. Choi, et al., Anal. Chem. 90 (2018) 2648-2654. DOI:10.1021/acs.analchem.7b04375 |
| [55] |
Y. Yang, H. Wang, Y.L. Wei, et al., Chin. Chem. Lett. 28 (2017) 2023-2026. DOI:10.1016/j.cclet.2017.08.051 |
| [56] |
Y. Kim, S.V. Mulay, M. Choi, et al., Chem. Sci. 6 (2015) 5435-5439. DOI:10.1039/C5SC02090E |
| [57] |
J. Liu, Y.Q. Sun, Y. Huo, et al., J. Am. Chem. Soc. 136 (2014) 574-577. DOI:10.1021/ja409578w |
| [58] |
G.X. Yin, T.T. Niu, Y.B. Gan, et al., Angew. Chem. Int. Ed. 57 (2018) 4991-4994. DOI:10.1002/anie.201800485 |
| [59] |
G. Yin, T. Niu, T. Yu, et al., Angew. Chem. Int. Ed. 58 (2019) 4557-4561. DOI:10.1002/anie.201813935 |
| [60] |
C.X. Yin, K.M. Xiong, F.J. Huo, et al., Angew. Chem. Int. Ed. 56 (2017) 13188-13198. DOI:10.1002/anie.201704084 |
| [61] |
Y. Yue, F. Huo, F. Cheng, et al., Chem. Soc. Rev. 48 (2019) 4155-4177. DOI:10.1039/C8CS01006D |
| [62] |
Y. Liu, J. Tan, Y. Zhang, et al., Biomaterials 173 (2018) 1-10. DOI:10.1016/j.biomaterials.2018.04.053 |
| [63] |
B. Shi, Z. Zhang, Q. Jin, et al., J. Mater. Chem. B 6 (2018) 7439-7443. |
| [64] |
F. Wang, Y. Zhu, L. Zhou, et al., Angew. Chem. Int. Ed. 54 (2015) 7349-7353. DOI:10.1002/anie.201502899 |
| [65] |
Y.Q. Sun, J. Liu, H. Zhang, et al., J. Am. Chem. Soc. 136 (2014) 12520-12523. DOI:10.1021/ja504156a |
| [66] |
H. Zhang, J. Liu, B. Hu, et al., Chem. Sci. 9 (2018) 3209-3214. DOI:10.1039/C7SC05407F |
| [67] |
X.X. Chen, L.Y. Niu, N. Shao, et al., Anal. Chem. 91 (2019) 4301-4306. DOI:10.1021/acs.analchem.9b00169 |
 2019, Vol. 30
2019, Vol. 30 




