b International Joint Research Center for Intelligent Biosensor Technology and Health, Key Laboratory of Pesticides and Chemical Biology, Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, China;
c State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang 550025, China
Noninvasive biomedical imaging has represented its superiority for allowing quick feedback of pathological and physiological processes in vivo opportunely. For the last decades, magnetic resonance imaging (MRI) and positron emission tomography (PET) with their FDA-approved probes (gadolinium-based contrast agents for MRI and fluorodeoxyglucose (FDG) for PET) are actively unearthed and promoted the development of clinical practices for disease diagnosis, monitor and prognosis. However, the above clinical applications are partly confined due to the imprecise location, inferior temporal and spatial resolution, high risk of dangerous radiation of ions, and etc. [1-3].
Owing to the high sensitivity, quick feedback, low cost and non-hazardous radiation, optical imaging has attracted extensive research attention from multidisciplinary areas such as chemical biology, analytic chemistry, materials science, biomedicine [4-19]. Optical imaginghas been divided into three channels: visible channel (400–700 nm), the first near-infrared channel (NIR-Ⅰ, 700–900 nm) and the second near-infrared channel (NIR-Ⅱ, 1000–1700 nm) (Fig. 1). Recent studies demonstrated that the NIR-Ⅱ fluorescence imaging opens a novel channel for precise diagnosis and prognosis with more reducing auto-fluorescence, decrease photoscattering, and less interfering absorption than the visible and NIR-Ⅰ channel [20-27]. Therefore, developing novel NIR-Ⅱ fluorophores for in vivo imaging applications thus has high significance and direct impact on the field of biomedicine. To date, nanoparticle-based fluorophores such as carbon nanotubes, Ag2S dots, rare-earth doped materials and conjugated polymers, have been actively employed [28-34]. On the other hand, small-molecule fluorophores remain to be the exceptional candidates for preclinical and clinical practice because of their high biocompatibility, favorable excretion pharmacokinetics and easy preparation. In this review, we focused on the development of small-molecule fluorophores and highlighted their multifunctional abilities in biomedical applications including the NIR-Ⅱ fluorescent imaging, multimodal imaging and theranostic.

|
Download:
|
| Fig. 1. Wavelengths for fluorescence imaging. Reproduced with permission [18]. Copyright 2019, John Wiley and Sons. | |
2. Small molecular NIR-Ⅱ fluorophores based on a benzobisthiadiazole core
Donor–acceptor–donor (D–A–D) structure is the most widely used scaffold in designing small molecular NIR-Ⅱ fluorophores. The combination of strong electron donors (D) and acceptors (A) is utilized to realize conjugate architecture (Fig. 2). Based on benzobisthiadiazole (BBTD) derivatives as a central acceptor group, a series of small molecules of D–A–D structure reduced the energy gap separating the hybridized highest occupied molecular orbital (HOMO)/lowest unoccupied molecular orbital (LUMO) levels and exhibited emission wavelength moved from the original NIR-Ⅰ region (700–900 nm) to the NIR-Ⅱ region (1000–1700 nm) [35-40].
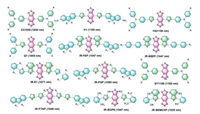
|
Download:
|
| Fig. 2. A library of small-molecule organic fluorophores based on a benzo-bisthiadiazole core and their max emission wavelength. | |
CH1055 as the foremost generation organic small molecular NIR-Ⅱ probe based on BBTD core was reported by Dai and Hong [34]. The conjugated system consists of a central BBTD moiety as the acceptor and two triphenyl amines flanked on both sides of BBTD as the donor group, resulting in its emission spectra bathochromic shift to ~1055 nm under the 808 nm excitation laser. Moreover, PEGylated CH1055 in aqueous solution showed super photo-stability than FDA approved NIR-Ⅰ dyes indocyanine green (ICG) and excellent excretion kinetics that ~90% probes were excreted through urine within 24 h. Excitingly, PEGylated CH1055 also highlighted sentinel lymph nodes (SLN) and lymphatic vasculature after an intradermal injection near the bottom of the tail and showed its double higher signal-to-background (S/B) ratio than that of ICG. All these advantages of this first molecular NIR-Ⅱ probe provide a positive potential for guiding accurate tumor/SLN removal surgery under NIR-Ⅱ imaging [34]. Based on the earliest NIR-Ⅱ small-molecule CH1055, a variety of novel organic fluorophores exhibited superior chemical and optical properties in NIR-Ⅱ channel. Typically, inspired by successfully constructed optical/PET dual modality imaging probes, the first NIR-Ⅱ/PET dual imaging probe 68Ga-CHS2 was concisely synthesized via base-catalyzed thiol-yne chemistry connecting CH1055 derivative and PET isotopes to an integrin αvβ3 targeted RGD peptide [40]. With the help of 68Ga-CHS2, the highest tumor-to-noise contrast which was 2-fold higher than traditional NIR-Ⅰ/PET RGD-based probe was determined at 12 h (T/N = 4.77). Furthermore, 68Ga-CHS2 demonstrated high spatial resolution in vivo for precise tumor delineation and real-time image-guided surgery [40]. As the quantum yield (QY) of CH1055 was lower than inorganic NIR-Ⅱ nanomaterials (0.3% vs. 15% for Ag2S quantum dots), increasing the QY of CH1055 based NIR-Ⅱ probes is very vital for in vivo applications. Based on this challenge, a bright NIR-Ⅱ probe CH-4T was designed via conjugation CH1055 with four taurine moieties. CH-4T showed impressively high QY (~5%) of the dye–protein complexes when the dye interacted with serum proteins of fetal bovine serum (FBS) and better temporal (50 FPS) and spatial (~1 μm) resolution than CH1055 [41].
Based on the scaffold of CH1055, Sun, Cheng and Hong constructed a series of small molecular probes Q1, Q4 and H1 each with a BBTD core successively (Fig. 2) [42-44]. Firstly, they compared the optical properties of Q1 (replaced a phenyl group of triphenyl amine with a thiophene based on CH1055) and Q4 (inserted a thiophene spacer between BBTD and triphenyl amine based on CH1055), Q4 demonstrated a longer emission wavelength and stronger fluorescence signals than Q1 on account of the electron-rich thiophene moiety exhibiting enhanced intramolecular charge transfer (Fig. 2) [42]. Then, they tried to substitute triphenyl amine of Q4 for 2-amino 9, 90-dialkyl-substitute fluorene to acquire a promising small molecular NIR-Ⅱ probe H1 with peak emission wavelength at ~1100 nm [44]. The fluorene moiety could tune the bandgap and the electrostatic potential distribution and the extended side chains on the 9, 90-position of fluorene could shield the conjugated backbone from intermolecular interaction, resulting in brighter fluorescence signals of H1 compared with Q4 (Fig. 2) [44]. Using hydrophilic polymer matrix such as DSPE-mPEG5000 to encapsulate hydrophobic small molecular probes is a wide method to increase water solubility and biocompatibility in vivo. By encapsulating H1 into DSPE-mPEG5000, H1 NPs was utilized in the distinct visualization of the vessels of the whole body and subcutaneous tumor and in image-guided surgery of removing SLN. In addition, based on the H1 scaffold, a newly tumor-specific targeting probe SDH, introducing the integrin αvβ3 targeting peptide RGD, was developed. SDH with specific NIR-Ⅱ imaging provided a noninvasive and real-time method to monitor early stage glioblastoma on U87MG tumor-bearing nude mice [44].
Considering the low QY of abovementioned small molecular NIR-Ⅱ probes, some inspirational and appealing strategies were proposed in response to improve fluorescence QY and enhance temporal resolution that brought about by high QY. Scientists found two ways to enhance QY of organic small molecule NIR-Ⅱ dyes. One way was mixed with serum to interact with serum proteins resulting in restricted of intramolecular rotation and coplanar molecular structures of dyes as dyes entered in protein hydrophobic domains [41]. Another way was adding shielding unit at the end of D–A–D structure to develop shielding-donor-acceptor-donor-shielding (S–D–A–D–S) architecture to distort the conjugated backbone and reduce intermolecular interactions. Dai group constructed a S–D–A–D–S structure firstly introducing 3, 4-ethoxylene dioxythiophene (EDOT) as the donor group between BBTD core unit and thiophene and 1, 2, 6-tri-substituted benzene as a shielding group with stretching side chains [45]. A high performance NIR-Ⅱ fluorophores IR-E1 was obtained with enhanced fluorescence QY (~0.7%) in aqueous solution. IR-E1 was applied in NIR-Ⅱ imaging and tracing cerebrovascular changes dramatically and noninvasively at single-vessel resolution in a traumatic brain injured (TBI) mouse model for the first time (Fig. 3A). To further demonstrate EDOT was a better donor group than thiophene, they compared IR-FE (EDOT as the donor unit) and IR-FT (thiophene as the donor unit). In addition, they substituted the fluorene to dibenzene to prepare a control IR-BBE. IR-FEP (PEGylated IR-FE) exhibited ~100 times higher QY (0.2% vs. 0.02%) in water and smaller hydrodynamic size owing to more diminished aggregation than IR-FTP (PEGylated IR-FT) and IR-BBEP (PEGylated IR-BBE) [46]. Based on S–D–A–D–S structure, S-D2-D1-A-D1-D2-S structure was constructed to extend conjugation length and increase bathochromic shift in response to improve optical properties of small molecular NIR-Ⅱ probes. Dai group investigated different functionalized thiophene as the first donor moiety connected to the second donor group thiophene with BBTD acceptor and fluorene shielding. Through theoretical calculation and practical measuring, IR-FTA, utilizing 3-alkyl substituted thiophene as the first donor, exhibited an extremely highest QY (5.3%) so far and better controlled synthesis and conjugation with biomolecules than existed inorganic NIR-Ⅱ fluorophores including quantum dots, SWCNT and rare earth nanoparticles. The success of IR-FTAP using in hindlimb vessel imaging brings the small molecular NIR-Ⅱ probes much closer to clinical translation [47]. Dai and coworkers developed a clickable NIR-Ⅱ probe IR-FGP, based on D–A–D structure, realizing multicolor 2D/3D imaging (Fig. 3B) in NIR-Ⅰ/Ⅱ dual-channel with other two kinds of conjugated antibody or protein fluorophores. The 3D layer-by-layer imaging displayed stained blood vessels, neuron and cell nucleus in three separate color channels between 850 nm and 1700 nm in one-photon confocal fluorescence imaging system [48].

|
Download:
|
| Fig. 3. (A) NIR-Ⅱ dynamic imaging of a TBI mouse and a sham mouse all injected with the IR-E1 fluorophore, resolving arterial (red) and venous cerebral vessels (blue). Reproduced with permission [45]. Copyright 2016, John Wiley and Sons. (B) Three-color 3D rendering of nucleus, neuron and vessel channels obtained with NIR-Ⅰ/Ⅱ confocal microscopy. Reproduced with permission [48]. Copyright 2017, National Academy of Sciences. (C) NIR-Ⅱ images of monitoring therapeutic response in HepG2 tumor bearing mice. (D) HepG2 tumor growth inhibition curves for PBS, cisplatin, and SY1100-based probe (diagnosis and treatment integration probe). Reproduced with permission [49]. Copyright 2019, National Academy of Sciences. (E) Image-guided surgery of HepG2 tumor. F) Comparison of NIR-Ⅱ images of the liver for the normal group and hepatic fibrosis group after injection of SCH4. Reproduced with permission [50]. Copyright 2018, John Wiley and Sons. | |
Based on the intrinsically optical and biocompatible merits of the NIR-Ⅱ small molecular probes, it is imperative to integrate therapeutic drugs and diagnostic fluorescent probes to realize real-time monitoring and assessment of the dynamic changes in tumor pathology during cancer treatment. Sun and Stang recently designed a novel NIR-Ⅱ fluorescent molecule SY1100 (emission wavelength of 1100 nm) with strong tissue penetrating ability and a macrocyclic platinum compound with significant antitumor activity and high stability in vivo. Based on the liposome technology, the first NIR-Ⅱ fluorescent probe based on the macrocyclic Pt structure for the diagnosis and treatment was prepared. Owing to the enhanced permeability and retention (EPR) effect, the probe could accumulate in tumors (Fig. 3C) and selectively release macrocyclic Pt compounds to achieve tumor inhibitory effects (Fig. 3D). In addition, due to the superior living stability of the nanoprobe, it could also evaluate the therapeutic effect of anti-tumor drugs by means of NIR-Ⅱ fluorescence imaging [49].
Though various sizes of above NIR-Ⅱ fluorescence probes were reported, it is still unknown whether the size of these probes influences their chemical/physical properties, particularly their optical/pharmacokinetic properties. Sun et al. employed an ingenious PEGylation strategy to precisely regulated the self-assembly size of CH1055 based NIR-Ⅱ probes SCH1 to SCH4 from single molecular size to nanoparticle size [50]. SCH4 with a minimum aqueous diameter of ~2 nm, showing high level of passive liver tumor uptake with promising S/N, was capable of facilitating image-guided tumor removing surgery (Fig. 3E) and also performed the first example of assessing liver fibrosis in living mice models in the NIR-Ⅱ region owing to its rapid excretion from renal system (Fig. 3F).
Recent years, some scientists made efforts on designing multifunctional molecular probe possessing both precisely imaging and effectively therapy properties, fabricating novel theranostics in terms of multi-modal imaging guided combined therapy on one small molecule [51, 52]. As a hybrid imaging modality, PA imaging integrated high contrast of optical imaging and high spatial resolution of ultrasound imaging, allowing for centimeterscale depth in living organisms that compensates the deficiencies of penetration depth of NIR-Ⅱ fluorescent imaging [53]. Cai and Gong added a nitro imidazole group on IR-1048 resulting in NIR-Ⅱ fluorescence, photoacoustic (PA) and photothermal signals were turn on through nitro group specifically responding to tumor hypoxic microenvironment. The "smart" probe could be sensitive to unique hypoxia condition, realizing precise tumor location and depiction through NIR-Ⅱ fluorescence/PA dual-modal imaging and noninvasive photothermal therapy (PTT) under 980 nm laser irradiation [54]. Fan et al. introduced a novel small molecule probe DPP-BDT, showing a maximum emission peak at 980 nm in the NIR-Ⅱ region when encapsulated in DSPE-mPEG5000. DPP-BDT NPs could achieve high resolution and deep penetration depth in the NIR-Ⅱ fluorescence and PA dual-modal imaging and image guided effective photothermal/photodynamic combination therapy under a single wavelength laser irradiation [55].
Taking advantage of combination with other modal imaging was a wise method to improve the quality of NIR-Ⅱ fluorescence imaging, additionally, reducing background interference through rapid clearance from blood vessels, skins and major organs was another benefited way. Rapid clearance also contributed to decreasing the cytotoxicity of exogenous molecule probes for desirable biocompatibility and biodegradation [56]. Liang et al. introduced a peptide-modified dye CP-IRT with renal excretion ability that ~87% of the probe could excrete from urine at 6 h postinjection [57]. Soon afterwards, they developed a NIR-Ⅱ fluorescence small molecule (IR-BGP6) that modified with the programmed cell death ligand-1 monoclonal antibody (PD-L1 mAb) which could profile the expression of a valuable biomarker programmed cell death ligand-1 (PD-L1) for immune checkpoint therapies. The compound realized fast renal excretion (~91% excretion within 10 h) with high tumor to normal tissue (T/NT) signal ratio [58]. Liang and Chen et al. used dialkoxy substituted benzene as shielding groups through sieving different substituent groups to construct a renal-excreted small molecule NIR-Ⅱ probe IR-BEMC6P with increased QY [56].
3. Small molecular NIR-Ⅱ fluorophores without a benzobisthiadiazole coreICG as the FDA approved clinically fluorescent contrast agent has been proven its safety through over 50 years of human experimentation and utilized for real-time fluorescence angiography for intraoperative assessment of arterial and venous flow, surgical guidance of removal of sentinel lymph nodes in tumor metastasis and monitoring tumor changes during treatment [59]. ICG exhibits 822 nm peak emission in the NIR-Ⅰ region, but it has long emission tails extending to the NIR-Ⅱ spectral region detected by InGaAs spectrometers (Fig. 4). Considering less autofluorescence, photo scattering and disturbing absorption at longer wavelengths in the NIR region, Annapragada et al. demonstrated that ICG exhibited an improved fluorescence emission above 1100 nm in plasma and higher contrast-to-noise ratios (CNR) in the NIR-Ⅱ window than the NIR-Ⅰ window at equivalent excitation flux [60]. Further experiment filling ICG in capillary tubes (to simulate vessels) embedded in 1% Intralipid plasma solution at different depths and the NIR-Ⅱ imaging demonstrated superior qualities ex vivo than the NIR-Ⅰ imaging using a home-built spectral NIR-Ⅰ/Ⅱ system. Meanwhile, Bruns et al. investigated the intravital application of ICG in more detail at clinically relevant doses in the NIR-Ⅱ channel [61]. Using an InGaAs camera instead of a traditional silicon camera (for NIR-Ⅰ imaging) recorded a low intensity of emission 1400 and 1500 nm (Fig. 5A). ICG exhibited notably enhanced image contrast for brain (Fig. 5B) as well as hind limb vasculature and microscopic imaging of the whole cranial window at different magnification compared with the NIR-Ⅰ image in vivo under 1300 nm LP filters. The exciting results demonstrated that under the NIR-Ⅱ channel the traditional NIR-Ⅰ small molecular probe ICG can represent its potential value in high background noise and label density with better image contrast. After that, they tried to inject IRDye 800CW (a commercial NIR-Ⅰ contrast agent also exhibits a broad emission spectrum expanding into the NIR-Ⅱ channel) conjugated with breast cancer-targeting antibody trastuzumab and PEGylated IRDye 800CW successively to obtained intravital multicolor fluorescence imaging under NIR-Ⅱ camera that behaving better imaging performance [62]. Among the existing NIR-Ⅰ dyes, Chen et al. compared fluorescence properties of several types of commercial NIR-Ⅰ dyes including cyanine dyes, CF dyes, Alexa Fluor dyes and DyLightBenzopyrillium dyes and found that cyanine dyes exhibited brilliant optical characteristic with extending tails in the NIR-Ⅱ region based on using the high detection efficiency of InGaAs detectors record the missing emission spectrum above 900 nm in the past [62]. Cyanine dyes contain two nitrogen groups and vinylene linkers between them. The NIR-Ⅱ emission spectrum of cyanine dyes was due to their characteristic chemical construction of the asymmetry of π domain in S1 state and the twisted intramolecular charge transfer (TICT). Due to cyanine dyes' S1 state is sensitive to surrounding environment, they can combine with serum proteins to improve fluorescence brightness when mixed with fetal bovine serum (FBS) or bovine serum albumin (BSA). In comparison to the NIR-Ⅰ dyes ICG and IRDye800, IR-12N3 showed 2–3 times enhanced fluorescence intensity in the presence of FBS. Then, imaging of brain and limb blood vessels through intact skull and skin, detecting the heart beats of the anesthetized mouse and lighting popliteal, sacral and lumbar lymph nodes (Figs. 5C and D) switching from 850 nm LP filters to 1300 nm LP filters resulted in super spatiotemporal resolution and signal contrast using IR-12N3 as contrast agent [62].
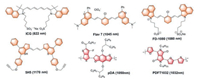
|
Download:
|
| Fig. 4. A library of organic fluorophores without a benzo-bisthiadiazole core and their max emission wavelength. | |

|
Download:
|
| Fig. 5. (A) The emission intensity of aqueous ICG solution, detected on an InGaAs camera (Inset shows the vial). (B) 850 nm LP NIR detection on a silicon camera and 1300 nm LP SWIR detection on an InGaAs camera. Reproduced with permission [61]. Copyright 2018, National Academy of Sciences. (C) Lumbar lymph nodes imaging at NIR-Ⅰ/Ⅱ windows. (D) Fluorescence cross-sectional intensity profile (red dashed line in (C)) of (C). Reproduced with permission [62]. Copyright 2018, John Wiley and Sons. (E) Absorbance and fluorescent emission spectra of FD-1080 dye (λabs = 1046 nm; λex = 1080 nm). Inset: An NIR-Ⅱ fluorescence image of FD-1080 (50 mm) in deionized water, FBS, and PBS under excitation of 1064 nm. (F) The respiratory rate of the awake (left) and anaesthetized (right) mouse. Reproduced with permission [64]. Copyright 2018, John Wiley and Sons. | |
Inspired by the cyanine dyes' structures, Cosco et al. engineered rational modifications on cyanine dyes by replacing the indolenines heterocycles with dimethyl-flavylium heterocycles and the resulting dyes have been used to visualize deep vasculature in living mouse [63]. Meanwhile, Zhang group designed a novel small molecular fluorophore FD-1080 with a cyanine core, both the absorption (1064 nm) and emission peak (1080 nm) fall in NIR-Ⅱ region (Fig. 4) [64]. Although the QY of FD-1080 was below 0.5% in organic solvent with IR-26 as reference (QY = 0.05%), FD-1080 and FBS complexion exhibited extremely high QY of 5.94% after incubation for 120 min at a heating temperature of 40 ℃ under 1064 nm excitation (Fig. 5E). The longtime incubation facilitated that the small molecular cyanine dye entered the hydrophobic pockets of a complex array of protein in FBS, resulting in the restricted intramolecular rotation (RIR) of FD- 1080 and concomitantly distinct improvement in fluorescence QY. Then, FD-1080 was utilized in realizing deep tissue hindlimb and brain vessels image with high temporal resolution, as well as quantifying the respiratory rate of the awake and anaesthetized mouse from the dynamic imaging data of respiratory craniocaudal motion of the liver (Fig. 5F). More recently, Hong et al. reported the first NIR-Ⅱa probe based on polymethinethiopyrylium fluorophores 5H5, which achieved significant image quality improvement under 1064 nm excitation (Fig. 4) [65]. Moreover, Yang et al. have designed and synthesized a new class of NIR-Ⅱ fluorophores based on bisbenzo-C-rodamine scaffold with excellent photostability [66].
In addition, several high extinction coefficient, superior photostability and ideal biocompatibility of D-A structured conjugated polymers (CPs) have been recently utilized in fluorescence imaging owing to the structure of donor-acceptor alternating copolymerization reducing the band gap between HOMO and LUMO and extending delocalized conjugated system through regulating various donor/acceptor moiety and the length of polymers. More and stronger electron donor/acceptor groups as well as longer polymers both lowered the band gap and broadened the conjugated plane resulting in emission spectra red-shift to the NIR-Ⅱ region with reducing wavelength-dependent scattering of photons [67-69]. For example, pDA polymer as the first example of NIR-Ⅱ CPs fluorophores, demonstrated fluorescence emission peak at ~1050 nm with a large Stokes shift of ~400 nm as well as an excellent QY of ~1.7%. Encapsulating pDA with phospholipid DSPE-mPEG non-covalently as a NIR-Ⅱ fluorophore achieved the first living cells imaging carried by CPs and recorded vascular velocity and cardiac cycle with high spatial-temporal (in the 10 μm-1 mm range and exceeding 25 frames per second) resolution [69]. Diketopyrrolopyrrole (DPP) and its derivatives as common electron acceptor groups, were frequently connected with diverse electron donor groups to construct CPs achieving biomedical imaging and amplified theranostics [70-72]. Considering DPP as an advantageous electron-deficient unit, Fan et al. designed a triblock conjugated polymer (POEGMA-b-PDPP-b-POEGMA) with fluorescence emission ~1073 nm in the NIR-Ⅱ region through easy sedimentation intrinsically circumventing multi synthetic steps. Besides favorable optical properties, POEGMA-b-PDPP-b-POEGMA was employed in photothermal therapy (PTT) to inhibit the growth of tumor owing to pronounced photothermal conversion efficiency of 52% [73]. After this, a semiconducting polymer PDFT32 based on furan-containing DPP polymers with a NIR-Ⅱ fluorescence emission peak at 1032 nm, was employed in completing real-time imaging-guide orthotopic osteosarcoma tumor resection surgery, evaluating the vascular embolization therapy in osteosarcomabearing mice and dynamic visualizing sentinel lymph node in the NIR-Ⅱ bio-window [74].
4. OutlookApart from existing successes, future work will focus on following tips from the level of overall situation.
a) Accelerating the progress of clinical translation. The low efficiency photon and poor photostability of ICG utilization in the NIR-Ⅱ channel still hampered its clinical use. Small-molecule fluorophores developed in NIR-Ⅱ possess plenty of advantages for medical utilization, indicating huge potentials for further practices. Before the clinical study in human subjects, the toxicology and pharmacokinetics are critical to be tested. More recently, the in vivo acute and chronic toxicity of CH1055-based probes has been just tested and cell proliferation, cell apoptosis and DNA damage should also be carried out in the future.
b) Exploring smart small-molecule fluorophores with longer absorption and emission wavelengths into NIR-Ⅱb channel. Currently, NIR-Ⅱ probes mostly lie in the NIR-Ⅱa regions and commonly designed without special responses to endogenous substance, however, by transferring their absorption or emission into the NIR-Ⅱb channel and chemically modifying them with certain functional groups, targeting and medical features can be established with lower disturbance of autofluorescence and higher penetration depth.
c) Due to an extreme difficulty for detecting brain diseases on clinical practices, current fundamental researches are devoted to develop high-efficiency probes for brain imaging. Although lots of organic materials have been used for brain network imaging, crossing BBB (blood-brain barrier) is still an obstacle for them to enter brain and light the structure and function of brain. Besides the injury of BBB, whether substances dispersing in the blood can enter the brain tissue from the capillaries of the brain depends on the physicochemical properties of invasive substances including lipid-water partition coefficient, molecular weight, surface charge and functionalization with special ligands that can mediate receptor-mediated transport [75]. Therefore, adjusting aforementioned properties of contrast agents can greatly improve the possibility to enter brain tissue for in situ imaging, diagnosis and therapy.
d) Driving the development of 3D fluorescence imaging. Though the imaging depth can be deepened under the NIR-Ⅱ region, the elaborate layer-by-layer physiological structure obviously cannot be realized with planar images. However, 3D imaging can finely address above problem and make full use of the deeper penetration of fluorescence imaging in the NIR-Ⅱ region.
AcknowledgmentsThis work was partially supported by grants from NKR&DPC (No. 2016YFD0200902), National Natural Science Foundation of China (No. 21708012), 111 Project (No. B17019), NSFHP (No. 2017CFB151), self-determined research funds of CCNU from the colleges, basic research and operation of MOE for the Central Universities (No.110030106190234), Wuhan Morning Light Plan of Youth Science and Technology (No. 201705304010321).
| [1] |
G. Hong, A.L. Antaris, H. Dai, Nat. Biomed. Eng. 1 (2017) 0010. DOI:10.1038/s41551-016-0010 |
| [2] |
F. Ding, S. Chen, W.S. Zhang, Y.F. Tu, Y. Sun, Bioorg. Med. Chem. 25 (2017) 5179-5184. DOI:10.1016/j.bmc.2017.08.034 |
| [3] |
D. Ni, E.B. Enlerding, W. Cai, Angew. Chem. Int. Ed. 58 (2019) 2570-2579. DOI:10.1002/anie.201806853 |
| [4] |
D. Yue, M. Wang, F. Deng, et al., Chin. Chem. Lett. 29 (2018) 648-656. DOI:10.1016/j.cclet.2018.01.046 |
| [5] |
D. Cheng, Y. Pan, B. Yin, L. Yuan, X. Zhang, Chin. Chem. Lett. 28 (2017) 1987-1990. DOI:10.1016/j.cclet.2017.08.021 |
| [6] |
X. Luo, J. Li, J. Zhao, et al., Chin. Chem. Lett. 30 (2019) 839-846. DOI:10.1016/j.cclet.2019.03.012 |
| [7] |
Z. Lei, Z. Zeng, X. Qian, Y. Yang, Chin. Chem. Lett. 28 (2017) 2001-2004. DOI:10.1016/j.cclet.2017.09.023 |
| [8] |
X. Luo, H. Yang, H. Wang, et al., Anal. Chem. 90 (2018) 5803-5809. DOI:10.1021/acs.analchem.8b00218 |
| [9] |
H. Chen, B. Dong, Y. Tang, W. Lin, Acc. Chem. Res. 50 (2017) 1410-1422. DOI:10.1021/acs.accounts.7b00087 |
| [10] |
H. Liu, L. Chen, C. Xu, et al., Chem. Soc. Rev. 47 (2018) 7140-7180. DOI:10.1039/C7CS00862G |
| [11] |
L. Wu, Y. Sun, K. Sugimoto, et al., J. Am. Chem. Soc. 140 (2018) 16340-16352. DOI:10.1021/jacs.8b10176 |
| [12] |
Y. Sun, X. Ma, K. Chen, et al., Angew. Chem. Int. Ed. 54 (2015) 5981-5984. DOI:10.1002/anie.201500941 |
| [13] |
Y. Xu, M. Tian, H. Zhang, et al., Chin. Chem. Lett. 29 (2018) 1093-1097. DOI:10.1016/j.cclet.2018.03.032 |
| [14] |
S. Long, Q. Qiao, L. Miao, Z. Xu, Chin. Chem. Lett. 30 (2019) 573-576. DOI:10.1016/j.cclet.2018.11.031 |
| [15] |
L. Yu, Y. Qiao, L. Miao, Y. He, Y. Zhou, Chin. Chem. Lett. 29 (2018) 1545-1559. DOI:10.1016/j.cclet.2018.09.005 |
| [16] |
H. He, T. He, Z. Zhang, et al., Chin. Chem. Lett. 29 (2018) 1497-1499. DOI:10.1016/j.cclet.2018.08.019 |
| [17] |
Z. Chen, Y. Xu, X. Qian, Chin. Chem. Lett. 29 (2018) 1741-1756. DOI:10.1016/j.cclet.2018.09.020 |
| [18] |
F. Ding, Y. Fan, Y. Sun, F. Zhang, Adv. Healthc. Mater. 8 (2019) 1900260. DOI:10.1002/adhm.201900260 |
| [19] |
W. Qin, C. Xu, Y. Zhao, et al., Chin. Chem. Lett. 29 (2018) 1451-1455. DOI:10.1016/j.cclet.2018.04.007 |
| [20] |
Y. Cai, Z. Wei, C. Song, et al., Chem. Soc. Rev. 48 (2019) 22-37. DOI:10.1039/C8CS00494C |
| [21] |
J. Li, K. Pu, Chem. Soc. Rev. 48 (2019) 38-71. DOI:10.1039/C8CS00001H |
| [22] |
Y. Fan, P. Wang, Y. Lu, et al., Nat. Nanotechnol. 13 (2018) 941-946. DOI:10.1038/s41565-018-0221-0 |
| [23] |
F. Ding, Y. Zhan, X. Lu, Y. Sun, Chem. Sci. 9 (2018) 4370-4380. DOI:10.1039/C8SC01153B |
| [24] |
J. Zhao, D. Zhong, S. Zhou, J. Mater. Chem. B 6 (2018) 349-365. |
| [25] |
W. Zhu, Sci. China Chem. 59 (2016) 203-204. DOI:10.1007/s11426-016-5556-5 |
| [26] |
H. Zhou, Y. Xiao, X. Hong, Chin. Chem. Lett. 29 (2018) 1425-1428. DOI:10.1016/j.cclet.2018.08.009 |
| [27] |
G. Hong, S. Diao, A. Antaris, H. Dai, Chem. Rev. 115 (2015) 10816-10906. DOI:10.1021/acs.chemrev.5b00008 |
| [28] |
Kenry, Y. Duan, B. Liu, Adv. Mater. 30 (2019) 1802394. |
| [29] |
C. Li, Q. Wang, ACS Nano 12 (2018) 9654-9659. DOI:10.1021/acsnano.8b07536 |
| [30] |
H. He, Y. Lin, Z. Tian, et al., Small 14 (2018) 1703296. DOI:10.1002/smll.201703296 |
| [31] |
Y. Li, S. Zeng, J. Hao, ACS Nano 13 (2019) 248-259. DOI:10.1021/acsnano.8b05431 |
| [32] |
P. Wang, Y. Fan, L. Lu, et al., Nat. Commun. 8 (2017) 14702. DOI:10.1038/ncomms14702 |
| [33] |
J. Qi, C. Sun, A. Zebibula, et al., Adv. Mater. 30 (2018) 1706856. DOI:10.1002/adma.201706856 |
| [34] |
A.L. Antaris, H. Chen, K. Chen, et al., Nat. Mater. 15 (2016) 235-242. DOI:10.1038/nmat4476 |
| [35] |
X. Zeng, Y. Xiao, J. Lin, et al., Adv. Healthc. Mater. 7 (2018) 1800589. DOI:10.1002/adhm.201800589 |
| [36] |
Z. Sheng, B. Guo, D. Hu, et al., Adv. Mater. 30 (2018) 1800766. DOI:10.1002/adma.201800766 |
| [37] |
J. Qi, C. Sun, D. Li, et al., ACS Nano 12 (2018) 7936-7945. DOI:10.1021/acsnano.8b02452 |
| [38] |
J. Yang, Q. Xie, H. Zhou, et al., J. Proteome. Res. 17 (2018) 2428-2439. DOI:10.1021/acs.jproteome.8b00181 |
| [39] |
J. Lin, X. Zeng, Y. Xiao, et al., Chem. Sci. 10 (2019) 1119-1126. |
| [40] |
Y. Sun, X. Zeng, Y. Xiao, et al., Chem. Sci. 9 (2018) 2092-2097. DOI:10.1039/C7SC04774F |
| [41] |
A.L. Antaris, H. Chen, S. Diao, et al., Nat. Commun. 8 (2017) 15269. DOI:10.1038/ncomms15269 |
| [42] |
Y. Sun, C. Qu, H. Chen, et al., Chem. Sci. 7 (2016) 6203-6207. DOI:10.1039/C6SC01561A |
| [43] |
K. Shou, C. Qu, Y. Sun, et al., Adv. Funct. Mater. 27 (2017) 1700995. DOI:10.1002/adfm.201700995 |
| [44] |
Y. Sun, M. Ding, X. Zeng, et al., Chem. Sci. 8 (2017) 3489-3493. DOI:10.1039/C7SC00251C |
| [45] |
X. Zhang, H. Wang, A. Antaris, et al., Adv. Mater. 28 (2016) 6872-6879. DOI:10.1002/adma.201600706 |
| [46] |
Q. Yang, Z. Ma, H. Wang, et al., Adv. Mater. 29 (2017) 1605497. DOI:10.1002/adma.201605497 |
| [47] |
Q. Yang, Z. Hu, S. Zhu, et al., J. Am. Chem. Soc. 140 (2018) 1715-1724. DOI:10.1021/jacs.7b10334 |
| [48] |
S. Zhu, Q. Yang, A. Antaris, et al., Proc. Natl. Acad. Sci. U. S. A. 114 (2017) 962-967. DOI:10.1073/pnas.1617990114 |
| [49] |
Y. Sun, F. Ding, Z. Zhou, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 1968-1973. DOI:10.1073/pnas.1817021116 |
| [50] |
F. Ding, C. Li, Y. Xu, et al., Adv. Healthc. Mater. 7 (2018) 1800973. DOI:10.1002/adhm.201800973 |
| [51] |
W. Wang, Z. Hu, Adv. Mater. 30 (2018) 1804827. |
| [52] |
Y. Sun, X. Ma, K. Cheng, et al., Angew. Chem. Int. Ed. 54 (2015) 5981. DOI:10.1002/anie.201500941 |
| [53] |
Y. Jiang, K. Pu, Adv. Biosyst. 2 (2018) 1700262. DOI:10.1002/adbi.201700262 |
| [54] |
X. Meng, J. Zhang, Z. Sun, et al., Theranostics 8 (2018) 6025-6034. DOI:10.7150/thno.26607 |
| [55] |
Q. Wang, B. Xia, J. Xu, et al., Mater. Chem. Front. 3 (2019) 650. DOI:10.1039/C9QM00036D |
| [56] |
R. Tian, H. Ma, Q. Yang, et al., Chem. Sci. 10 (2019) 326-332. DOI:10.1039/C8SC03751E |
| [57] |
W. Wang, Z. Ma, S. Zhu, et al., Adv. Mater. 30 (2018) 1800106. DOI:10.1002/adma.201800106 |
| [58] |
H. Wan, H. Ma, S. Zhu, et al., Adv. Funct. Mater. 28 (2018) 1804956. DOI:10.1002/adfm.201804956 |
| [59] |
C.A. Metildi, S. Kaushal, M. Pu, et al., Ann. Surg. Oncol. 21 (2014) 1405-1411. DOI:10.1245/s10434-014-3495-y |
| [60] |
Z. Starosolski, R. Bhavane, K.B. Ghaghada, et al., PLoS One 12 (2017) e0187563. DOI:10.1371/journal.pone.0187563 |
| [61] |
J.A. Carr, D. Franke, J.R. Caram, et al., Proc. Natl. Acad. Sci. U. S. A. 115 (2018) 4465-4470. DOI:10.1073/pnas.1718917115 |
| [62] |
S. Zhu, Z. Hu, R. Tian, et al., Adv. Mater. 30 (2018) 1802546. DOI:10.1002/adma.201802546 |
| [63] |
E.D. Cosco, J.R. Caram, O.T. Bruns, etal., Angew.Chem.Int.Ed. 56 (2017) 13126-13129. DOI:10.1002/anie.201706974 |
| [64] |
B. Li, L. Lu, M. Zhao, Z. Lei, F. Zhang, Angew. Chem. Int. Ed. 57 (2018) 7483-7487. DOI:10.1002/anie.201801226 |
| [65] |
B. Ding, Y. Xiao, H. Zhou, et al., J. Med. Chem. 62 (2019) 2049-2059. DOI:10.1021/acs.jmedchem.8b01682 |
| [66] |
Z. Lei, X. Li, X. Luo, et al., Angew. Chem. Int. Ed. 56 (2017) 2979-2983. DOI:10.1002/anie.201612301 |
| [67] |
Y. Wang, Q. Peng, Q. Hou, et al., Theor. Chem. Acc. 129 (2011) 257-270. DOI:10.1007/s00214-011-0932-x |
| [68] |
K.G. Jespersen, W.J. Beenken, Y. Zaushitsyn, et al., J. Chem. Phys. 121 (2004) 12613-12617. DOI:10.1063/1.1817873 |
| [69] |
G. Hong, Y. Zou, A.L. Antaris, et al., Nat. Commun. 5 (2014) 4206. DOI:10.1038/ncomms5206 |
| [70] |
Y. Hang, X.P. He, L. Yang, J. Hua, Biosens. Bioelectron. 65 (2015) 420-426. DOI:10.1016/j.bios.2014.10.058 |
| [71] |
H. Chen, J. Zhang, K. Chang, et al., Biomaterials 144 (2017) 42-52. DOI:10.1016/j.biomaterials.2017.08.007 |
| [72] |
Y. Cao, J. Yi, X. Yang, et al., Biomacromolecules 18 (2017) 2306-2314. DOI:10.1021/acs.biomac.7b00464 |
| [73] |
X. Lu, P. Yuan, W. Zhang, et al., Polym. Chem. 9 (2018) 3118-3126. DOI:10.1039/C8PY00215K |
| [74] |
K. Shou, Y. Tang, H. Chen, et al., Chem. Sci. 9 (2018) 3105-3110. DOI:10.1039/C8SC00206A |
| [75] |
H. Wang, X. Mu, J. Yang, et al., Coord. Chem. Rev. 380 (2019) 550-571. DOI:10.1016/j.ccr.2018.11.003 |
 2019, Vol. 30
2019, Vol. 30 

