b Key Laboratory of Pesticide and Chemical Biology, Ministry of Education; Hubei International Scientific and Technological Cooperation Base of Pesticide and Green Synthesis; International Joint Research Center for Intelligent BiosensingTechnology and Health; College of Chemistry, Central China Normal University, Wuhan 430079, China;
c State Key Laboratory of Chemical Oncogenomics, Key Laboratory of Chemical Biology, the Graduate School at Shenzhen, Tsinghua University, Shenzhen 518055, China
Photodynamic therapy (PDT) [1-4] is a promising noninvasive modality for the treatment of tumors by combining a light-excited photosensitizer, oxygen and light. Upon irradiation with light of long wavelength, the photosensitizer undergoes intersystem crossing (ISC) and rapidly forms the triplet excited states. The triplet-excited state energy is then transferred to ground state (triplet) molecular oxygen to generate singlet-excited state of oxygen (singlet oxygen), which is the key cytotoxic agent of PDT.
Compared to other conventional treatment protocol such as chemotherapy, radiotherapy and surgery, PDT has several excellent advantages. Firstly, PDT is applicable to all kinds of tumors. Secondly, the treatment time of PDT is short and it is a non-toxic and non-immunity therapy [5]. More importantly, PDT can be used alone as well as in combination with other therapeutic modalities, including radiotherapy, chemotherapy, surgery, photothermal therapy (PTT), gene therapy or immunotherapy. In addition to these advantages, PDT also possesses some disadvantages. On the one hand, PDT has poor treatment effect in the tumor microenvironment because it is an oxygen-dependent process [6]. On the other hand, light with short wavelength cannot penetrate tissue, and since the generation of singlet oxygen requires the necessary energy, it is less effective in promoting PDT. As a result, the research of fluorescence agents with wavelengths in the range of 650– 850 nm is important for PDT.
In the past few years, many molecules have been reported for PDT, especially porphyrins. Porphyrins played an important role in developing photosensitizers and luminescence markers. Porphyrins possessed the characteristic of producing singlet molecular oxygen with high quantum yields after photoexcitation, which could kill tumor cells effectively. Nowadays, numerous porphyrinderivatives have been prepared for PDT, based on substitution pattern of the chromophore and inner heavy atom. Recently a series of halogenated and sulfonated porphyrins have been reported in anticancer therapies by Dąbrowski's group [7]. Compared with hydrophilic and anionic porphyrins, porphyrin derivatives reported above were taken up by A549 and CT26 cells more quickly and easily with cell mortality of 90%. The most common inner metal atom is palladium and platinum. Wiehe et al. [8] reported five palladium(Ⅱ) porphyrins exhibiting only a very weak fluorescence and generating singlet molecular oxygen with a quantum yield (~0.85). The result of phototoxicity in Jurkat cells showed that cell vitality was only 5%–10% after irradiation. Furthermore, some inorganic atoms have been developed to replace the metal atom in view of water solubility. Matsumoto et al. [9] synthesized a series of phosphorus porphyrin complexes irradiated by light emitting diodes (LEDs) which showed high photodynamic activity in cancer cells. Moreover, the use of LEDs provided a homogeneous light distribution for the target cells with higher PDT efficiency. With the development of fluorescence imaging technology [10-14], the design of fluorescent dyes with good properties has always been the focus of researchers. And In order to achieve the effect of diagnosis and treatment, the design of fluorescent probes [15-17] for PDT has also become a research hotspot. In this review, we provide a concise overview of small organic molecules used in PDT in recent years. The structures of the organic small molecular dyes and the main data are shown in Schemes 1–4, Figs. 1–5 and Tables 1–4.
|
Download:
|
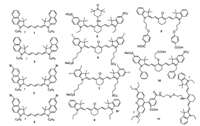
| Scheme1. The structures of cyanine-based photosensitizers. | |

|
Download:
|
| Scheme2. The structures of BODIPY-based photosensitizers. | |

|
Download:
|
| Scheme3. The structures of phtalocyanines-based photosensitizers. | |

|
Download:
|
| Scheme4. The structures of other agents-based photosensitizers. γ-GGT: γ-glutamyltranspeptidase. | |
|
Download:
|
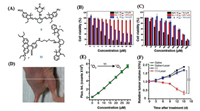
| Fig. 1. (A) The structures of 5 and 11. (B) Phototoxicity evaluation of PpIX, Dye 1 and 5 on HeLa cells for 20 min irradiation (λex = 660 nm, 50 mW/cm2), MTT assay was measured after another 6 h incubation. (C) Dark cytotoxicity evaluation of PpIX, Dye 1 and 5 on HeLa cells. (D) The photo of nude mouse bearing MCF-7 tumor. (E) The linear relationship between fluorescent intensity of DCF. (F) The growth curve of MCF-7 xenograft tumors within 15 days in different groups. (B and C) Reproduced with permission [25]. Copyright 2018, Royal Society of Chemistry. (D–F) Reproduced with permission [31]. Copyright 2017, Ivyspring International Publisher. | |
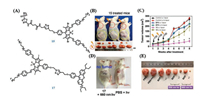
|
Download:
|
| Fig. 2. (A) The structures of 15 and 17. (B) (Top) Representative images of nude mice 8 weeks after intravenous tail vein injection of 15 followed by 660 nm laser irradiation (2.0 W/cm2, 30 min; 3600 J/cm2) of the upper tumor (red circle). The lower tumor (yellow circle) was used as an on irradiated control. (Bottom) Dissected tumors from each group. (C) Tumor volume of the mice in the BPS or 15 groups with or without PDT treatment. (D) Representative photos of mice with 17 and PBS under light laser at the 14th day. (E) Representative tumor images from different groups of tumor-bearing mice. (B and C) Reproduced with permission [38]. Copyright 2017, American Chemical Society. (D and E) Reproduced with permission [40]. Copyright 2018, American Chemical Society. | |
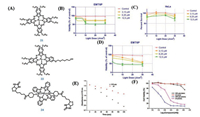
|
Download:
|
| Fig. 3. (A) The structures of 21, 22 and 24. (B) In vitro phototoxicity (EMT6P cells) of 21 at 3 h after incubation. (C) In vitro phototoxicity (HeLa cells) of 21 at 3 h after incubation. (D) In vitro phototoxicity (EMT6P cells) of 22 at 3 h after incubation. (E) Comparison of the rate of photodegradation of DPBF using 24 (5 μmol/L) under irradiation with red light. (F) Cytotoxicity against HeLa cells of 24 in the dark or upon irradiation. (B–D) Reproduced with permission [45]. Copyright 2016, Wiley Online Library. (E and F) Reproduced with permission [47]. Copyright 2017, Elsevier. | |
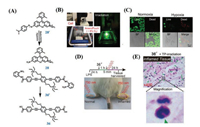
|
Download:
|
| Fig. 4. (A) The structures of 28, 28', 36 and 36'. (B) Construction of a mildly hypoxic environment (~8% O2) with an AnaeroPouch-MicroAero. (C) Live/Dead staining images of A549 cells. Cells were photoirradiated under normoxia or hypoxia. (D) Illustration for in vivo 36' of inflamed mouse model. (E) Histological H & E assay for tissues at 24 h after the treatment with 36' under TP-irradiation at 750 nm for 5 min. (B and C) Reproduced with permission [53]. Copyright 2017, American Chemical Society. (D and E) Reproduced with permission [67]. Copyright 2017, Royal Society of Chemistry. | |

|
Download:
|
| Fig. 5. (A) The structure of 41 and 42. (B) Photodynamic therapy set-up. (C) Tumor images for each group. (D) Average tumor volumes of each group of mice bearing H1299 tumor. (E) Presence of light irradiation under normoxia (21% O2) or hypoxia (2% O2) conditions. (F) Relative tumor volume of mice after different treatments. (B–D) Reproduced with permission [69]. Copyright 2017, Elsevier. (E and F) Reproduced with permission [70]. Copyright 2018, American Chemical Society. | |
|
|
Table 1 The data of cyanine-based photosensitizers. |
|
|
Table 2 The data of BODIPY-based photosensitizers. |
|
|
Table 3 The data of phtalocyanines-based photosensitizers. |
|
|
Table 4 The data of other agents-based photosensitizers. |
2. Cyanine-based photosensitizers
NIR fluorescent heptamethineindocyanine dyes preferentially accumulate in tumor cells and function in the wavelength range of 700–900 nm [18-23], in which the content of intrinsic chromophores in native tissue is extremely low to visualize tumors sensitively and noninvasively in vivo. This kind of dyes also show photodependent cytotoxicity, suggesting potential therapeutic efficiency for tumor targeting, imaging, PTT and PDT. Among cyanine dyes, indocyanine green (ICG) has been recognized as a commercial dye by FDA and explored as a potential photosensitizer. In this section, we briefly summarize the structures and the data of cyanine dyes used in PDT (Scheme 1 and Table 1).
Expect for ICG, more and more cyanine dyes have been reported for photoexciation cancer therapy like PDT. Barbero et al. [24] reported four novel NIR pentamethine cyaninedyes 1–4 as potential photosensitizers for PDT. These dyes were modified with bromine on the benzoindolenine ring, C4 or C2 alkyl chains, which improved singlet oxygen production and enhanced PDT mediated cytotoxicity. All dyes exhibited a narrow absorption band in the NIR range in different solvents, matching the phototherapeutic window perfectly. The light-induced cytotoxic effect on HT-1080 cell of each cyanine dye suggested that the presence of bromine had no remarkable influence on the photodynamic effect. In addition, all the tested cyanine dyes showed an obvious phototoxicity at very low concentration, making them suitable and appealing in vivo. In 2018, Peng et al. [25] developed a water-soluble NIR aminocyanine dye 5 (Fig. 1A) with a long triplet-state lifetime (τ = 9.16 μs in deaerated ethanol) for PDT in tumor region, which could generate cytotoxic reactive oxygen species (ROS) to induce cancer cells death. The author designed the structural skeleton of Cy7 dyes labelled 2, 2, 6, 6-tetramethylpiperidinyloxy (TEMPO), which was known to efficient ISC process for triplet-state photosensitizers. The dye 5 possessed a large stokes shift (~100 nm) due to the introduction of 4-amino-TEMPO at the center of the conjugated bridge. Fortunately, the absorption and fluorescence peaks were both in NIR range, which madeit suitable for bio-applications.The PDT potential of dye 5 was affirmed by nanosecond time-resolved transient difference absorption spectra. A salient feature of transient absorption was found between 630 nm and 820 nm after 532 nm laser excitation. And the two bleaching peaks were located at 660 nm and 790 nm respectively. The long-living transient was found to be sensitive to oxygen, which indicated that efficient radical-enhancedISC occur in dye 5 due to the radical substituent, thereby the dye 5 obtained a long excited triplet state. Enhanced ISC by the introduced TEMPO group for dye 5 should be attributed to electron spin polarization (ESP) based on a radical-triplet pair mechanism (RTPM). The singlet oxygen generation abilities of dye 5 were measured by using 1, 3- diphenylisobenzofuran (DPBF) as a singlet oxygen trapping agent. The absorbance of DPBF at 410 nm decreased remarkably in the presence of dye 5 under irradiation. The singlet oxygen quantum yield (ΦΔ) of dye 5 was calculated by choosing methylene blue in ethanol with FDMB = 0.52 as reference. The MTT assays of dye 5 showed that it had an obvious phototoxicity and low dark cytotoxicity (Figs. 1B and C). Acridine orange (AO) stained the DNA and RNA of living cells and gave out green fluorescence. Ethidium bromide (EB) stained DNA and RNA of dead cells and gave out red fluorescence. The cells' AO/EB staining suggested that cells death caused by PDT was predominantly via apoptosis but not necrosis. Furthermore, it obviously demonstrated dye 5 had a bright prospect for biomedical and clinical applications in the future.
Early in 2012, Atchison et al. [26] designed a new class of iodinated cyanine dyes used in NIR excited PDT based on the structure of IR-783, a commercially available NIR cyanine dye with similar absorption and emission properties to ICG but had a modified chemical structure. The absorption peak of 6 with monoiodinated was 790 nm and the absorption of 7 with di-iodinated was much broader with a significantly blue shift at 680 nm. After excitation at 775 nm for ICG and 6, and 687 nm for 7, it revealed a significantly quenched emission for 7 when compared to either ICG or 6. Moreover, the singlet oxygen generation yield of 6 (ΦΔ = 0.66) and 7 (ΦΔ = 0.44) irradiated with 780 nm light (100 mW) showed 7.9 folds and 4.4 folds higher than that of ICG, respectively. The compounds had PDT effect in MIA PaCa-2 cell line and BxPC-3 cell line. Interestingly, the effect was more dramatic in the MIA PaCa-2 cell line with less than 10% viable cells remaining at 50 μmol/L drug concentration while the value was approximately 40% for BxPc-3 at the same concentration. Whereafter, 6 was selected as a candidate for in vivo studies. 6 was intra-tumorally injected in mice bearing tumors and showed excellent PDT effect.
In order to improve the elimination effect of tumor, researchers constructed a diagnosis and treatment preparation combining PDT and PTT based on the properties of cyanine dye. ICG is a photothermal agent, photosensitizer, and fluorescence imaging probe which shows specific accumulation in hepatocellular carcinoma (HCC) cells. The group of Shirata developed a photosensitizer using ICG and NIR laser as a new anti-cancer treatment for HCC [27]. ROS production was detected after NIR irradiation both in vitro and in vivo. Besides, ICG was excited by 823-nm NIR light, and approximately 88% of the absorbed light was converted to heat. ICG produced heat up to 48.5 ℃ during NIR light irradiation in vivo.
Other cyanine dyes had been used as photosensitizers. Chen et al. [28] synthetized compound 8, a new mitochondria-targeting NIR fluorescent small molecule, which possessed structureinherent PDT and PTT synergistic phototherapeutic effects without conjugation to specific ligands. The generation of singlet oxygen was detected by singlet oxygen sensor green (SOSG). The fluorescence intensity of SOSG in compound 8 was remarkably higher than blank water, which indicated 8 had the great PDT efficacy. A549 and LLC cells were dead after incubating with compound 8 and being excited with 808 nm laser irradiation (2 W/cm2). More importantly, the PTT and PDT synergistic effects were testified by the mice bearing LLC cells. In 2016, Luo and his coworkers designed a variety of heptamethine cyanine dyes [29], which were modified with various N-alkyl side chains on the lipophilic cationic heptamethine core. Among these compounds, compound 9 was proved to be the best mitochondria-targeted NIR photosensitizer for synchronous PDT and PTT. The ROS generation of 9 was detected by SOSG. Upon NIR laser irradiation, the singlet oxygen production of 9 was higher than that in blank water and the temperature of compound 9 increased sharply. Furthermore, the therapeutic effect of 9 was demonstrated by A549 cells and the subcutaneous tumor xenograft models upon the laser irradiation. Gu and her coworkers introduced a new multifunctional small molecule fluorescent dye 10 used for synchronous long-duration cancer imaging, PDT and PTT [30]. 10 could be used to optical image in vivo due to a quite strong fluorescence at 820 nm under the excitation of 765 nm. So under 808 nm laser (0.5 W/cm2) for 5 min, 10 led to an obvious ROS generation and more efficient ROS was generated under enhanced laser intensity. The temperature of 10 increased rapidly above 50 ℃, whereas ICG increased only in the first 3 min. 10 had different functions with different light irradiation. Under the guidance of the 765 nm laser-induced fluorescence of 10 accumulated on the tumor tissue, another NIR laser (808 nm) could be accurately focused on the tumor tissue for phototherapy. 10 showed superior photostability and phototherapy properties. Meanwhile, 10 significantly improved the phototherapeutic efficiency for U87MG tumor-bearing mice upon light laser and the highest survivor ratio was observed because of spontaneous tumor targeting and simultaneous synergetic PTT and PDT. Further, liver and intestine were the main metabolic organ of 10.
Currently, versatile small-molecule therapeutic probes used for tumor targeted imaging and precision therapy have been given more and more attention. Based on this strategy, Meng et al. [31] reported a small-molecule theranostic probe, 11 (Fig. 1A), with "Four in One" functions including thiols/pH sensing, tumor targeting, NIR fluorescence and photoacoustic (NIRF/PA) dual-modal imaging and PDT, which was synthesized by conjugating 5'-carboxyrhodamines (Rho) with heptamethinecyanine IR765 (Cy) via a disulfide linker and pH tunable amino-group. Although the absorption peaks of Rho moiety and Cy moiety are 550 nm and 640 nm, respectively, the absorption peak of 11 was at 480 nm and 640 nm. In addition, 11 has two emission peaks at 580 nm and 765 nm when excited at 480 nm. Interestingly, 11 may have fluorescence resonance energy transfer (FRET) from the excited state of Rho moiety to Cy moiety because of a good spectral overlap between the emission spectrum of the Rho (550–630 nm) and absorption spectrum of the Cy (580–700 nm). The fluorescence intensity of 11 at 580 nm increased and the drop of emission peak at 765 nm (λex = 480 nm) was observed at the same time when GSH concentration increased. In NIR range, the fluorescent intensity of 11 at 765 nm (λex = 640 nm) increased greatly as the pH value of probe solution decreased owing to the amino connected to Cy moiety. 11 had natively preferential tumor accumulation property because of Cy. Based on the characteristics of 11, it was used as a dual-modal NIRF/PA imaging. The 11 has Cy moiety with potential photocytotoxicity for PDT. 11 can immediately produce ROS in the solution with 660 nm laser light at 30 mW/cm2 power, and the ROS levels increased with the increase of 11's concentration by measuring the fluorescence intensity using dichlorofluorescein (DCF) assay (Fig. 1E). It is important that the ROS levels produced by 11 were highly efficient and even higher than chlorin e6 (Ce6), a second-generation photosensitizer with high ROS yield at the same concentration. Then MCF-7 cells were stained by 11 upon light irradiation and the cell viability reduced to 20% when the concentration of 11 was 20 μmol/L. For therapeutic efficacy of 11 in vivo, the survive rate of 11-treated mice with laser irradiation was also extensively improved to 80%, while saline-treated mice with laser is only 10%. Therefore, the well-defined 11 as a dual-responsive small-molecule probe and theranostic agent may have important applications in tumor targeted imaging and precision therapy (Figs. 1D and F).
3. BODIPY-based photosensitizersThe BODIPY chromophore [32-34] has gained substantial attention. BODIPY dyes have been attractive as photosensitizers due to their excellent optical properties, high molar extinction coefficient, excellent photostability and generally high fluorescence quantum yields, and thus have been applied as imaging/ detection agents. We introduce some photosensitizers based on the structure of BODIPY (Scheme 2). We also summarize the data of BODIPY used in PDT (Table 2).
Conventional BODIPY photosensitizers have been synthesized by increasing spin orbit coupling through the heavy atom effect. Many reports indicated that the inclusion of heavy atoms, such as bromine and iodine, show cytotoxicity in the absence of light and compromise fluorescence yield due to increased ISC efficiency. Early in 2016, Kim and cooperator developed a halogenated BODIPY dye (12) [35]. 12 in acetonitrile exhibited sharp steadystate peak of absorption and fluorescence emission at 528 nm and 546 nm, respectively, with a fluorescence quantum yield (ΦF) of 0.02. 12 exhibited characteristic luminescence of singlet oxygen (1O2) at 1270 nm by a direct measurement of NIR luminescence with a ΦΔ of 0.93. The high 1O2 quantum efficiency of the dye could be attributed to the introduction of two iodine atoms at the 2- and 6-positions, which enhanced the ISC efficiency from an excited singlet state to an excited triplet state. LLC cells were stained with 12, and cell viability remained over 70% until 15 μmol/L while cell viability decreased to 50% at a dye concentration of 10 μmol/L with a light dose of 3.5 mW/cm2, which proved 12 was an efficient light-induced toxicity of the agent with good cellular biocompatibility.
It is well-known that lysosome is an important cellular organelle. The lysosomal membrane destabilization will lead to proton and hydrolase leakage, resulting in organelles dysfunction and even cell necrosis, apoptosis, and pathological symptoms. Hence, designing lysosomes targeted PDT agents can rapidly induce dysfunction of lysosomes and then destroy cancer cells. In 2017, Li et al. [36] reported a diiodo-substituted BODIPY, 13, which showed an intense peak of absorption at 660 nm. Upon excited at 660 nm, 13 showed a fluorescence emission at 694 nm with an absolute ΦF of 0.11.The ΦΔ of 13 was calculated as 0.64 by the relative method using MB as the reference (ΦΔ = 0.57 in dichloromethane) in the presence of illumination with 660 nm red light. 13 showed no inhibition of growth of MCF-7 cells in dark and across all agent concentrations, as expected.Moreover, PDT efficacy positively correlated with both the delivered 13 dose and light dose, which demonstrated that 13 can observably damage cells after light laser. The half-maximal inhibitory concentration (IC50) of 13 was lower than that of the commercial photosensitizer, Ce6. 13 penetrated into cells and primarily located in lysosomes because of the existence of morpholine. Lysosomes can receive and degrade unwanted materials from outside of the cell and obsolete components inside the cell with the aid of numerous acid hydrolases. To maintain the optimal activity of hydrolases, lysosomes dwell in the acidic microenvironment because of the proton-pumping vacuolar ATPase. So except for conjugation of lysosomes-targeted group, pH-responsive photosensitizer can specific response to lysosomes of cancer cells for PDT. Based on this strategy, the group of Xue designed and synthesized an amino modified BODIPY compound [37], 14', used as a smart pH-response photosensitizer. 14' showed moderated emission at 553 nm only in acidic conditions owing to the protonation of diethylamino but the emission at 553 nm was decreased by a factor of 14 from pH 3.0 to 5.5 when the pH value was increased. The absorption of 14' from 450 nm to 600 nm showed no obvious change with pH because of photo induced electron transfer. Under 532 nm laser irradiation, the ΦΔ of 14 at pH of 3.5 and 5.5 was 0.45 and 0.24, respectively, indicating a more efficient singlet oxygen production of 14 in more acidic conditions. For cell imaging, 14' accumulated in the lysosomes in both HePG-2 and HL-7702 cells. After then, normal cells and cancer cells were treated with 14' upon photoirradiation, and the cell viability of normal cells (>90%) was unchanged while cell viability of cancer cells was dramatically decreased after extended irradiation, which illustrated that 14' can be specifically activated by lysosomes for PDT and had minimal toxicity in normal cells.
A major challenge in PDT is avoiding PDT-induced hypoxia, which can lead to cancer recurrence and progression through activation of various angiogenic factors and significantly reduce treatment outcomes. Jung et al. [38] reported an acetazolamide-conjugated BODIPY photosensitizer (15) (Fig. 2A) designed to mitigate the effects of PDT-based hypoxia by combining the benefits of antiangiogenesis therapy with PDT. 15 had an intense absorption band at 661 nm and a fluorescence emission feature centered around 689 nmwith the ΦF of 0.06 upon excitation at 660 nm. The ΦΔ of 0.6 was calculated for 15, which are in line with what is observed for several photosensitizers in clinical use, such as MB. Importantly 15 was found to work well and be stable in PBS media containing 10% DMSO under conditions of photoirradiation. Interestingly, 15 could target carbonic anhydrase IX (CAIX), which was subject to tumorspecific overexpression with highly restricted expression within normal tissues. 15 treated the CAIX/high cancerous MDA-MB-231 cells and the CAIX/low cancerous MCF-7 cells. The MDA-MB-231 cells showed strong fluorescence intensity compared to MCF-7 as expected. The higher uptake level of 15 was confirmed by confocal imaging, which illustrated that 15 could accumulate in special tumor. Upon laser irradiation of the MDA-MB-231 cells at 660 nm (2.0 W/cm2, 30 min) incubated with 15 (5 μmol/L), about 92.5% of cell population was seen to be the stages of late apoptosis or necrosis. Finally, 15 was injected in the mice bearing MDA-MB-231 xenografts and then irradiated at 660 nm. And the tumors were excised, stained with anti-CD31 antibody (an angiogenesismarker), and subject to cryosection. Tumors analyzed in this way exhibited significantly decreased expression of CD31, which suggested that hypoxia-induced upregulation of CAIX induced a series of proteins associated with neo-angiogenesis. 15 could provide a benefit because of the combination of two modalities, namely, PDT and anti-angiogenesis, which gave new design concept in areas of cancer diagnosis and treatment (Figs. 2B and C).
All the time, researchers have been working to achieve precision treatment, and various specific protein receptors have been found, such as above mentioned CAIX. Tropomycin kinase receptor C (TrkC) has been identified as a characteristic regulator of breast cancer cell growth and metastasis and tends to be overexpressed in metastatic breast tumor cells. Consequently, it is a potential target for active targeting of metastatic breast cancer. Yang and co-workers developed a novel compound (16) based on BODIPY used as a photosensitizer and the bivalent targeting fragment used as a NIR probe for TrkC targeted imaging [39]. 16 showed the greater photocytotoxicity for TrkC + breast cancer cell lines HS578 t and 4T1. It was remarkable that 16 at 10 mg/kg caused on average 96% tumor volume reduction in the mice bearing TrkC + tumor at day 6 postPDT. 16 had a PDT effect, but short-term toxicities of 16 were a concern because, while doses of 20 mg/kg were tolerated, 30 mg/kg were not. And 16 should require further structural modifications because the light wavelength to excite this was optimally around 520 nm whereas PDT agents should absorb above 700 nm if they were to be addressed at more than 1 cm tissue penetration. In any event, 16 provided a prototype for developing novel photosensitizers by targeting tumor with TrkC overexpression.
Structure-inherent targeting (SIT) agents are of particular importance for clinical precision medicine. Hence in 2018, Li et al. [40] proposed an innovative strategy for using the FRET mechanism to devise robust SIT phototheranostics (17) (Fig. 2A) for significantly amplifying the therapeutic outcome. The structure of 17 was synthesized by being connected with presentative cationic rhodamine moiety used as an energy donor to pair up with a photosensitizer, diiododistyrylbodipy. 17 displayed not only a typical Q-band in the range of 600–700 nm, but also possessed an additional intense absorption peak centered at 557 nm, which belonged to the rhodamine moiety. Upon 568 nm excitation, the fluorescence of 17 at 575 nm was completely quenched while an emission peak was at 650 nm with a shorter fluorescence lifetime, proving that efficient energy transfer had taken place in 17. 17 was also a singlet oxygen generation agent upon photoexcitation with light source from 400 nm to 700 nm. Then 4T1 cells were stained with 17 and 17 could rapidly internalize the tumor cells, illustrating that 17 had excellent cellular uptake character. With 12 J/cm2 light irradiation (660 nm), the IC50 of 17 was merely 0.036 μmol/L and the cell viability of 4T1 cells was less than 20%, which suggested that 17 showed a better PDT effect than other photosensitizers such as Ce6. Interestingly, when 4T1 cells were exposed to a 550 nm light source, almost no cells were killed by BDP in all doses.17 was intravenously injected into the BALB/c mice bearing a subcutaneous 4T1 tumor, the fluorescence intensity of 17 at the tumor site gradually enhanced over time, which was much higher than that at adjacent muscle tissues. At the same time, 17 displayed the inhibition of tumor invivo upon 550 nm or 660 nm light irradiation. As expected, 17 was an excellent photosensitizer upon different light irradiation and enabled the PDT in confined tumor regions so as to reduce the side effects on normal tissues (Figs. 2D and E).
The inclusion of heavy atoms, such as bromine and iodine, had advantages for PDT, but showed cytotoxicity in the absence of light and compromise on fluorescence yield due to increased ISC efficiency. Thus, BODIPY photosensitizers without heavy halogen atoms are preferred for effective dual clinical applications. Early in 2015, Watley et al. [41] discovered a series of fused BODIPY dyes for PDT, 18, without heavy halogen atoms. The absorption peak of 18 was at 690 nm and displayed a fluorescence emission at 700 nm, which could be a good potential as a NIR fluorescence dye, although the small stokes shift (10 nm) could be a problem. The ΦΔ of 18 was 0.42 after light laser. Colon- 26 cells were treated with 18 upon photoirradiation, and cell mortality was more than 50%. Finally, tumor-bearing BALB/c mice were used to demonstrate therapeutic potential in vivo. 18 with photoirradiation showed a dramatic reduction in tumors compared to other control groups and mice were pronounced cured after tissue remodeling with no palpable tumor observed at 60 days post treatment, which illustrated that 18 was an excellent photosensitizer. In 2016, the group of Shivran developed a new water-soluble BODIPY dye, 19, possessing a glycosylated styryl moiety at the C-3 position by a simple synthetic route [42]. 19 had absorption and emission at the range of 550–600 nm with the high fluorescence quantum yield. 19 showed impressive photosensitization property against A549 cells, and ROS-mediated apoptosis via the caspase-8/caspase-3-dependent extrinsic pathway with low IC50. 19 did not show any dark toxicity to human normal cell and cancer (A549 cells) and non-toxic to the normal lung cells even on photo-exposure.
4. Phtalocyanines-based photosensitizersPhthalocyanines (Pc) [43] fulfill many essential requirements of efficient sensitizers for PDT. Up to now, several Pc derivatives have been extensively studied as notable second-generation photosensitizers in PDT applications due to their suitable physical and chemical properties. Common phthalocyanine dyes included zinc (Zn) Pcs and silicon (Si) Pcs, which can be used in PDT (Scheme 3). The data of phthalocyanines-based photosensitizers is shown in Table 3.
ZnPcs present intense fluorescence in the NIR region which renders them excellent photosensitizers for fluorescence imaging method that has been used to diagnose cancer. And it is known that radiolabeled Pc can be used as nuclear imaging agents. In 2016, Avsar et al. [44] synthesized ZnPc, 20, bearing four binaphtyl groups in order to image and investigate cytotoxicity of PDT. Based on the UV–vis absorption of DPBF as the scavenger, 20 was revealed to be an efficient singlet oxygen generator (ΦΔ = 0.612). In vitro fluorescence imaging study with MCF-7 cells showed that 20 localized in cytoplasm of the cells. These results showed that synthesized 20 was a promising candidate for dual fluorescence/nuclear imaging breast cancer and showed the potential photosensitizer for PDT application. Although the results were still preliminary, further detailed In vivo investigations were also necessary in breast tumor implanted animals to clarify the potential of 20 for fluorescence/nuclear imaging agent. In the same year, Lambrecht et al. studied multifunctional agents, 21 and 22 (Fig. 3A), used in both nuclear imaging and PDT [45]. The bio-distribution of 21 in normal female rats indicated high uptake in the large intestine and stable uptake in the ovary. However, the uptake of 22 was significant in the large intestine, pancreas and ovary. In addition, 21 and 22 demonstrated good PDT efficacy for a mouse mammary cell line. And 21 and 22 were effective for PDT on EMT6/P and HeLa (Figs. 3B–D). The 21 might be an agent for nuclear imaging of ovary and colon tumors, while 22 might be useful for ovary, pancreatic and colon tumors. Further investigations were also necessary in tumor implanted animals to clarify the usability of 21 and 22 for tumor imaging and PDT.
Mitochondrion is considered quite appealing as it can be as a biological target. Early in 2011, the group of Zhao reported the synthesis of a mitochondria-targeting PDT agent, 23, by covalently connecting a well-known mitochondria-targeting agent, namely rhodamine B, to a tumor-localizing photosensitizer and evaluated its subcellular localization, one- and two-photon PDT properties [46]. The simultaneous appearance of rhodamine and SiPc characteristic absorption in the spectra of 23 confirmed their rhodamine-SiPc conjugate structure. And the absorption of 23 had two peaks at 557 nm and 680 nm. Interestingly, for 23, excitation of the rhodamine moiety band at 515 nm led to emissions from both the rhodamine and the Pc moieties while excitation of the Pc chromophore at 640 nm resulted in emission exclusively from the Pc moiety. Using the singlet oxygen quantum yield of tetraphenylporphyrins (ΦΔ = 0.55 ± 0.11) as the reference standard, the relative ΦΔ of 23 was 0.48 ± 0.11 upon photoexciation at 549 nm. At the same time, SiPc of 23 was the main moiety of generating singlet oxygen and linking two rhodamine moieties could increase the ΦΔ of SiPc. 23 had two-photon absorption property and the two- photon absorption cross sections of 23 were derived to be 1494 GM at 850 nm. As for cell experiment, HK-1 cells were costained with 23 and mitochondria-specific probe. Then the mitochondria-specific probe and 23 were found to localize in the mitochondria of HK-1 cells. The cell vitality of HK-1 cells treated with 100 nmol/L of 23 was reduced to 20% by photoirradiation at 4 J/cm2. After excited at 850 nm, 23 had significant two-photon induced cytotoxicity for PDT.
Up to now, various tumor targeting ligands have been explored and employed to develop novel photosensitizers with improved selectivity in cancer cells. Among these tumor targeting vectors, biotin, also known as vitamin H, is a promising agent for targeted delivery of therapeutic or diagnostic drugs and recognized to be essential for cell growth, and particularly for the tumor cells, which requires greater amounts of biotin than the normal cells to sustain their rapid proliferation. In 2017, Li et al. [47] designed and synthesized a novel photosensitizer, 24 (Fig. 3A), by axial conjugation of SiPc with two biotin molecules which could enhance the selective accumulation of photosensitizers in biotin receptor overexpressed cancer cells. The peak of UV–vis absorption of 24 was at 674 nm and the fluorescence emission peak at 686 nm upon excitation at 610 nm. The ΦΔ of 24 was also measured by using DPBF as a scavenger and the ΦΔ of 24 was 0.43 (Fig. 3E). Since biotin receptors are overexpressed in HeLa cells, 24 was significantly higher associated in HeLa cells. 24 could selectively damage biotin receptors positive cancer cells and decreased the cell viability of HeLa cells to 20% under irradiation (Fig. 3F). Additionally, 24 was a safe and effective photosensitizer in clinical application because of low dark toxicity toward L02 cells. At the same year, Gülmez et al. [48] also reported photosensitizers, 25 and 26, by connection with SiPc and axially bis- and mono-biotin. Upon light irradiation, 25 and 26 showed similar singlet oxygen generation (ΦΔ = 0.36 for 25 and 0.35 for 26). Then in vitro cell studies indicated that the studied 25 and 26 had a cytotoxic effect on HeLa cells, the highest decrease in viability was observed at 10 μmol/L concentration with 2 J/cm2 light dose. The novel SiPcbiotin conjugates 25 and 26 which synthesized in this study are promising suitable photosensitizers for PDT applications.
Integrin αvβ3, a transmembrane glycoprotein, is overexpressed on blood vessels in tumors and in some cancer cell lines like HT-29, A549 and HeLa cells, but not in normal tissues, and it can be used as a target for the early diagnosis and treatment. Based on above description, it has been well documented that the peptides with an arginine-glycine-aspartic acid (RGD) sequence showed high affinity to integrin αvβ3. Zheng et al. reported a novel photosensitizer, 27, by conjugating SiPcs with two cyclic RGD (cRGD) through ethylene glycol linkers, which exhibited highest selectivity toward HT-29 cells overexpressed integrin αvβ3 [49]. 27 displayed the intense UV–vis absorption peak at 672 nm, which was the typical UV–vis absorption peak for non-aggregated phthalocyanines. Upon excitation at 610 nm, 27 showed a fluorescence emission at 679 nm with a ΦF of 0.40. And the ΦΔ of 27 was 0.32. Then HT-29 human colon carcinoma cells with high expression of integrin αvβ3 and MCF-7 human breast adenocarcinoma cells with low expression of integrin αvβ3 were incubated with 27. Obviously for 27, the IC50 values of MCF-7 cells was higher than that toward HT- 29 cells, suggesting 27 with two cRGD showed selectivity toward HT-29 cells overexpressed integrin αvβ3. The cell viability of HT-29 cells decreased to less than 20%. To future study the phototoxicity of 27, KM mice bearing a H22 tumor, a tumor with high expression of αvβ3 integrin, was treated with 27. 27 could preferentially accumulate in tumor tissue of tumor-bearing mice and showed a significant PDT effect resulting in 75% tumor growth inhibition.
5. Other photosensitizersExcept for cyanines, BODIPYs and Pcs, some novel structures were reported and used as photosensitizers (Scheme 4). And then we summarize the basic data of these structures (Table 4). Rhodamine [50-52], a classical fluorescent dye, has been widely used as a fluorophore moiety because of its excellent photophysical and chemical properties. And rhodamine and its derivatives have been widely concerned and used in the research of new generation photosensitizers. Recently, various chromophores containing a selenium atom have been utilized for probes and photosensitizers due to effective ISC by the internal heavy atom effect. In 2017, Piao et al. [53] developed a photosensitizer that was activated under hypoxic conditions, 28 (Fig. 4A), by introducing an azo moiety into the conjugated system of a selenorosamine dye, a kind of rhodamine derivatives. Compared to 28 without azo moiety, 28' showed a red-shifted and broader absorption spectrum. The maximum absorption peak was at 630 nm, but no emission was observed. Using rose bengal (ΦΔ = 0.75) as a standard, the ΦΔ values of 28 and 28' were calculated as 0.56 and 0.03, respectively, which indicated that singlet oxygen production was suppressed in 28' because of hindrance of ISC by the azo group. Interestingly, 28' was discovered that it can be activated selectively under hypoxia and released 28, which had the emission peak at 557 nm. In order to prove that 28 had excellent PDT effect under hypoxia, A549 cells were incubated with 28' under either normoxia or hypoxia. After the photoirradiation, A549 cells were destroyed with 28' under hypoxia and the cell viability was reduced to 40%. Importantly, 28 could induce cell damage at ~8% oxygen concentration (Figs. 4B and C). Except for hypoxia activation, tumor specific peptidase activation was a strategy for the sake of more aggregation in cancer. The group of Chiba adopted a spirocyclization-based strategy to design γ-glutamylhydroxymethyl selenorhodamine green as a photoinactive compound, 29', that could be cleaved by the tumorassociated enzyme γ-glutamyltranspeptidase (GGT) to generate the potent photosensitizer 29 [54]. The absorption and emission peak of 29' was at 534 nm and 562 nm, respectively. And 29 showed the pH-dependent change in absorption spectrum. As expected, γ-glutamyl derivatives of 29' displayed little singlet oxygen production upon 532 nm laser irradiation. In order to examine whether 29' could induce cell death in a GGT activitydependent manner, 29' was cultured with high GGT activity of SHIN3 cells and low GGT activity of SKOV3 cells after light irradiation. SHIN3 cells with the treatment of 29' showed a strong fluorescence and excellent cell damage, which illustrated that cell death was induced regardless of GGT activity. Then 29' treated to the different chick chorioallantoic membrane (CAM), a widely used model for evaluating drug toxicity and angiogenesis inhibitors. CAM implanted with the high GGT activity of A549- Lus-C8 spheroids by the treatment of 29' was destroyed but CAM as a normal tissue model at the same condition did not show obvious death, which suggested that 29' had highly specific phototoxicity toward GGT expressing tumors without damage to surrounding tissue. In 2012, Chou et al. [55] developed a fluorescent molecule 3, 6-bis(1-methyl-4-vinylpyridinium) carbazolediiodide, 30, for PDT. Importantly, the fluorescence of 30 detected in cancer cells was much stronger than that in normal cells, suggesting it to be a good candidate for a tumor-targeting agent. The maximum absorption peak of 30 was shifted from 435 nm to 460 nm and the fluorescence intensity increased significantly when 30 interacted with the DNA of cancer cells at about 570 nm. The TC-1 cell viability of 30 (20 μmol/L) decreased to ~60% upon light irradiation at 445 nm and photo-induced cytotoxicity of 30 increased with the increasing of light dose. Further experiments showed that 30 plus light significantly inhibited the growth of tumor cells in vivo. Therefore, 30 was used as a potential molecule for PDT of cancer cells.
Traditional photosensitizers, such as porphyrin, with large planar structures show aggregation-caused quenching (ACQ) with weak fluorescence and reduced singlet oxygen generation in solid state or in aggregates due to π-π stacking. So there is an increasing interest in development of photosensitizers with aggregation induced emission (AIE) characteristic [56-58]. In 2015, the group of Zhang designed and synthesized mitochondria targeting AIE photosensitizers, which were combined with tetraphenylethylene and triphenylphosphonium (TPP) [59]. The peak of UV–vis absorption of 31 and 32 were at 410 nm. The photoluminescence (PL) spectra of 31 and 32 increased when the volume fraction of hexane is gradually increased to more than 80% and the nano-aggregates formation was also confirmed by laser light scattering (LLS), which illustrated that these photosensitizers had AIE characteristic. Meanwhile, these compounds could effectively drive the accumulation to mitochondria through colocalization experiment. Under white light illumination, 31 and 32 showed strong inhibition of cell viability with longer irradiation time at higher irradiation power. Besides, different from 31, 32 with two TPP groups was able to exert chemo-cytotoxic in the dark. So the probe of 32 was a new generation of subcellular targeted theranostic agent with multifunction, such as cancer cell detection, imaging, PDT, and chemotherapy without direct drug conjugation. In 2016, Yuan et al. [60] developed a bioprobe, 33, composed of photosensitizer with AIE property and rhodol dye conjugated via a singlet oxygen cleavable aminoacrylate (AA) linker. At the same time, 33 had higher tumor cellular uptake concentration because of cRGD. 33 was used as photosensitizer and imaging agent, which has a shoulder peak at 430 nm and emission maximum at 650 nm. The ΦΔ of 33 was determined to be 0.68 using rose bengal (RB) as the standard photosensitizer (ΦΔ =0.75 in water), which was much higher than traditional photosensitizers such as photofrin (ΦΔ = 0.28). The AIE property of 33 was confirmed by PL spectra. Interestingly, the fluorescence signal of Rho moiety showed a quick and steady increase upon light irradiation, which confirmed that the AA linker can be selectively cleaved by single oxygen. Hence, 33 was not only a new photosensitizer, but also can be used for real-time self-monitoring of singlet oxygen generation during PDT.
There are some new structures used as AIEgens expect for tetraphenylethylene. Recently Wang and co-workers reported a water-soluble NIR AIEgen, 34, which was a phototherapeutic agent and also the first fluorescent "light up" probe for cell imaging with a short staining process [61]. It was significantly enhanced emissions of 34 with increasing the fraction of tetrahydrofuran due to formation of aggregations. 34 displayed maximum absorption bands at 480 nm and emission peak in aggregation state at 708 nm, with 1.7% quantum yields owing to the connection of electron-accepting units with electron-donating units via p-bridge, which illustrated that 34 had AIE characteristic. In addition, the high ΦΔ (80.16%) for 34 was detected by using a common singlet oxygen probe 9, 10-anthracenediyl-bis(methylene) dimalonic acid (ABDA), as the indicator, and employed RB as the standard photosensitizer.
To verify PDT effect of 34, HeLa cells with treatment of 34 upon light irradiation were studied. It was observed that the viability of these HeLa cells dropped to 15% with a concentration of 500 nmol/L, indicating 34 had remarkable efficiency for cancer cell ablation in the PDT pathway. Subsequently, 34 clearly provided tumor imaging and cancer ablation after intratumoral injection of 34 in HeLa tumor-bearing mice. Importantly, 34 maintained an "off" state in an aqueous environment. And thus 34 was an excellent ultrafast staining probe for plasma membrane with minimal background interference from both free dyes and biosubstrate autofluorescence. Zou et al. [62] reported a probe, 35, by conjugation of a FR/NIR AIEgen with TPP, as a multi-functional fluorophore for mitochondrial imaging and PDT. The fluorescence spectrum of 35 ranged from 560 nm to 800 nm with a maximum emission at 662 nm. The fluorescence intensity of 35 in DMSO solution was very weak. However, the fluorescence intensity gradually enhanced with the increase of water fractions. As for PDT, the probe generated ROS with high ROS quantum yield of 65% under white light irradiation (4 mW/cm2). High efficient ROS generation of 35 was good for improving PDT efficiency. A549 cells was co-stained with 35 (50 nmol/L) and MTG (50 nmol/L), a commercial mitochondrial dye, respectively, which was studied that 35 had a clear image of the mitochondria. 35 exhibited good phototoxicity, which could kill approximately 80% of A549 cells. Zebrafish was used as an important vertebrate model because approximately 70% of human genes were orthologous to those in zebrafish. The bioimaging results further suggested that 35 could target mitochondria precisely and be used as a wonderful photosensitizer in living systems and zebrafish embryos.
Elevated nitric oxide (NO) level performs an important pathological role in various inflammatory diseases. Hence, developing NO-activatable theranostic materials with two-photon excitation feature [63-66] is highly promising for precision imaging and therapy. In 2017, Hu and co-works presented a rational design to a NO-activatable fluorescence photosensitizer, 36' (Fig. 4A), accompanying with NO-elevated property for realizing efficient two-photon imaging and PDT [67]. 36' was synthesized with a zwitterionic bis(phenylethynyl)benzene derivative, which exhibited an ultrahigh two-photon absorption crosssection. 36' displayed a broadband one-photon absorption spectrum with excellent molar extinction coefficient at 361 nm and a very weak fluorescence center at 415 nm with ΦF of 0.17%. Upon the addition of NO, the absorption maximum almost kept unchanged while ΦF of 36' exhibited 18-folds enhancement from 0.17% to 9.3% at 415 nm A series of experiments explained that 36' had high selectivity toward NO. And 36' possessed maximum twophoton absorption cross-section of 3300 GM at 710 nm upon responding to NO. Two-photon imaging of 36' in HeLa cells was studied and 36' was found a strong fluorescence with approximately 5 min after being added a NO generator. 36' was injected in an inflamed mouse model in which inflammatory lumps in right rear paw, then a distinct fluorescence enhancement in inflamed tissues in comparison with that of normal tissues, which was consistent with previous conclusions. Finally, the ΦΔ of 36 in the environment of NO was 82% and the cell viability of activated macrophages incubated with 36' was gradually reduced to 19.1% under two-photon irradiation at 750 nm, which preliminarily demonstrated that 36' could serve as a smart theranostic agent for a precise therapy (Figs. 4D and E).
Recently, the group of Zheng described a series of far-red and NIR AIE luminogens, 37–40, with a strong push-pull effect by connecting electron-donating diphenylamine group to four different electron-withdrawing groups [malononitrile, isophorone, methylpyridinium salt, and 3-cyano-4-phenyl-2(5H)-furanone] through an electron-rich carbazolyl ring [68]. 37–40 showed their absorption maximum at 480, 491, 528, and 496 nm, respectively. And 37–40 had NIR solid-state fluorescence with a high quantum yield of up to 30% and large stokes shifts up to 244 nm because of AIE characteristics. This series of compounds displayed excellent two-photon absorption activity in the range of 800–1040 nm. Impressively, 39 exhibited a maximum two-photon absorption cross-section of 887 GM at 1020 nm. Soon afterwards, the bright fluorescence signals of compounds incubated with HeLa cells could be observed, which suggested a fast permeability of AIEgens for living cells. 37–40 had also excellent organelle specificity. The excellent specificity of 37–39 to stain lipid droplets (LDs) could be attributed to the highly selective accumulation of the lipophilic AIEgens in the hydrophobic spherical LDs thanks to the "like-like" interactions. 40 firstly stained the mitochondria because of the cationic lipophilic property. Because two-photon excitation and NIR emission were especially important for optical in vivo deep imaging, these compounds were used as imaging probes of tissue with deep tissue penetration (up to 150 μm) and high contrast. 37–40 had both PDT effect, especially 40. The cell viability of 40 showed a sharp drop to 10% with a concentration of 5 × 10-6 mol/L, which illustrated that 40 was superior to Ce6, a commercial photosensitizer.
Quinacridon (QA) derivatives usually displayed strong absorption and high photoluminescent efficiency in dilute solution and good photothermal effect. Hence, the group of Liu designed a QA derivative in biological application [69]. 41 (Fig. 5A) was mixed with 2′, 7′- dichlorodihydrofluorescein diacetate (DCFH-DA) as the fluorescence probe, and the fluorescence of DCFH was high after irradiation under a 532 nm laser for 30 s because of the high ROS generation of 41. The data of cell experiment was consistent with that in solution, which indicated that 41 could generate ROS under irradiation not only in solution but also in cells. Interestingly, the cell viability decreased to 30% after only 10 s irradiation owing to photoinduced DNA cleavage, further confirming the strong photocytotoxicity of 41. After then, 41 was tail intravenously injected in mice and aggregated in tumor, which confirmed that 41 could target tumors in vivo. The tumor of mice with the treatment of 41 and light irradiation did not show noticeable change while that tumor in control groups grew rapidly within 16 days, which suggested that 41 had high photoactivated antitumor effect (Figs. 5B–D).
Hypoxia has been considered as one of the major factors of tumor microenvironment. But it also limited treatment outcome and poor prognosis for PDT because that tumoricidal effect relied highly on the oxygen level. Recently, to fight the negative effect of hypoxia, Li et al. [70] developed a NIR light-activated molecular O2-·generator with Nile blue analogs modified with "heavy atom", 42 (Fig. 5A). 42 displayed an intense absorption and emission profile in the NIR window, which promised deeper permeability and less phototoxicity to normal organisms. Encouragingly, 42 enabled to generate sufficient O2-·under light irradiation via Type I photoreaction under hypoxic environment (2% O2. Given the strong O2-·generation potency, 42 incubated with HepG2 cells was exposed to 660 nm light under hypoxic and normoxic environment (21% O2) by standard droethidium (DHE) staining assay. The average fluorescence intensity increased from 82 to 2146 folds as the light dose processed from 0 to 14.4 J/cm2 in HepG2 cells in the normoxic environment. And remarkable DHE fluorescence for O2-· was still detected in HepG2 cells, implying 42 might have satisfactory PDT activity under hypoxia (Fig. 5E). Up to 94% cancer cells were killed with a low concentration of 42 (0.63 μmol/L). Interestingly, the formed O2-·of 42 could not only serve as oxidant to kill cancer cells, but also participate in superoxide dismutase (SOD)-mediated reaction to form H2O2 under acidic condition and its downstream highly toxic OH· by a Haber-Weiss reaction or Fenton reaction, which improved anticancer effect. As a result, strikingly selective PDT effect was achieved on cancer in vivo (Fig. 5F) and 42 could be easily metabolized through kidney.
6. ConclusionIt has witnessed dramatic developments in PDT owing to the feasibility and efficacy of this approach to cancer treatment. Many of photosensitizers such as Ce6 [71] and porphyrins [72] had also been approved for clinical trial or use. In order to advance PDT effect, nanoparticle-based photosensitizers [73-76] has now become fascinating tools and hot topics of research owing to some advantages, including integration of multiple imaging, reduced toxicity and passive accumulation at cancer by the enhanced permeability and retention effect. However, some scientific and industrial challenges impede the development of nanoparticle-based photosensitizers such as complexity, uncontrollability, instability and uncertain nanomaterial toxicity. Based on this condition, the small molecule probes [77-84] that can be easily synthesized and controlled could address the troubles of nanomaterials. In this review, we summarized the small molecule fluorescent dyes used in PDT in recent years based on the classification of structures. It can be found that, compared with mature photosensitizers like Ce6, these structures show better single-line oxygen generation capacity. In addition, these dyes also have some other properties, such as two-photon absorption, specific targeting, synergistic therapy.
Although the researches on the application of organic small molecular dyes in PDT have been continued, there are still some problems that limit the application of small molecules in clinical PDT. Firstly, due to differences in the internal and external environment, the amount of functional singlet oxygen generated by small molecule photosensitizer is not controllable. Therefore, it is of great significance to quantitatively define functional singlet oxygen level, especially in type I PDT. Secondly, the tumor has an inherent hypoxic environment, and this special microenvironment seriously influences the PDT effect. Therefore, it is important to pursue innovative hypoxia-confronted PDT systems that have robust biocompatibilities. Finally, translating the novel PDT agents into clinical applications for the destruction of deep-seated tumors is also an important research hotspot. We expect that the utility of small molecule fluorescent dyes should grow in the near future with the further rapid development of small molecules and the addressing of the adverse factors of small molecule dyes used as photosensitizers.
AcknowledgmentsWe are grateful to the Natural Science Foundation Committee of China (NSFC, No. 81671803), the National Key Research and Development Program (No. 2017YFC0107700), the Outstanding Youth Foundation of Jiangsu Province (Nos. GX20171114003, BK20170030), Fok Ying Tung Education Foundation (No. 161033), "Double First-Class" University Project (Nos. CPU2018GY06 and CPU2018GY24) and the Priority Academic Program Development of Jiangsu Higher Education Institutions for their financial support. Yin acknowledges the National Natural Science Foundation of China (Nos. 21676113, 21402057, 21772054, 21472059), Distinguished Young Scholar of Hubei Province (No. 2018CFA079) for the financial support. This work is also supported by the 111 Project (No. B17019).
| [1] |
K.R. Rong, Z.Y. Hou, J. Lin, et al., Small 13 (2017) 1702299. DOI:10.1002/smll.201702299 |
| [2] |
X. Li, Z. Liu, J. Yoon, et al., Angew. Chem. Int. Ed. 57 (2018) 11522. DOI:10.1002/anie.201805138 |
| [3] |
F. Bolze, S. Jenni, V. Heitz, et al., Chem. Commun. 53 (2017) 12857-12877. DOI:10.1039/C7CC06133A |
| [4] |
J. Zhang, C.S. Jiang, H. Zhang, et al., Acta Pharmacol. Sin. B 8 (2018) 137-146. DOI:10.1016/j.apsb.2017.09.003 |
| [5] |
Z.Y. Sun, L.P. Zhang, F.P. Wu, et al., Adv. Funct. Mater. 27 (2017) 1704079. DOI:10.1002/adfm.201704079 |
| [6] |
X.S. Li, S.Y. Lee, J. Yoon, Chem. Soc. Rev. 47 (2018) 1174-1188. DOI:10.1039/C7CS00594F |
| [7] |
B. Pucelik, R. Paczynski, G. Dubin, et al., PLoS One 12 (2017) e0185984. DOI:10.1371/journal.pone.0185984 |
| [8] |
A. Wiehe, H. SGtollberg, S. Runge, et al., J. Porphyrins Phthalocyanines 5 (2001) 853-860. DOI:10.1002/jpp.553 |
| [9] |
J. Matsumoto, K. Suzuki, M. Yasuda, et al., Bioorg. Med. Chem. 25 (2017) 6536-6541. DOI:10.1016/j.bmc.2017.10.031 |
| [10] |
Y.H. Chen, T.W. Wei, Z.J. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1957-1960. DOI:10.1016/j.cclet.2017.05.010 |
| [11] |
X.X. Ma, L.L. Hu, X. Han, et al., Chin. Chem. Lett. 29 (2018) 1489-1492. DOI:10.1016/j.cclet.2018.06.022 |
| [12] |
C.J. Reinhardt, E.Y. Zhou, M.D. Jorgensen, et al., J. Am. Chem. Soc. 140 (2018) 1011-1018. DOI:10.1021/jacs.7b10783 |
| [13] |
W.J. Chen, Y.L. Pan, J.H. Chen, et al., Chin. Chem. Lett. 29 (2018) 1435-1439. |
| [14] |
P.Z. Zhang, Z.Q. Guo, C.X. Yan, et al., Chin. Chem. Lett. 28 (2017) 1952-1956. DOI:10.1016/j.cclet.2017.08.038 |
| [15] |
Y. Zhao, M.M. Cerda, M.D. Pluth, Chem. Sci. 10 (2019) 1873-1878. DOI:10.1039/C8SC05200J |
| [16] |
S.L. Leng, Q.L. Qiao, Y. Gao, et al., Chin. Chem. Lett. 28 (2017) 1911-1915. DOI:10.1016/j.cclet.2017.03.034 |
| [17] |
Y. Kang, J.L. Fan, Q. Jin, et al., Chin. Chem. Lett. 28 (2017) 1991-1993. DOI:10.1016/j.cclet.2017.08.054 |
| [18] |
Z.W. Yuan, L.J. Gui, H.Y. Chen, et al., ACS Appl. Mater. Interfaces 10 (2018) 30994-31007. DOI:10.1021/acsami.8b09841 |
| [19] |
J.R. Zheng, Y.Z. Shen, Z.Q. Xu, et al., Biosens. Bioelectron. 119 (2018) 141-148. DOI:10.1016/j.bios.2018.08.014 |
| [20] |
L.J. Gui, Z.W. Yuan, H.Y. Chen, et al., Chem. Commun. 54 (2018) 9675-9678. DOI:10.1039/C8CC04246B |
| [21] |
Z.Q. Xu, X.T. Huang, X. Han, et al., Chemistry 7 (2018) 1609-1628. |
| [22] |
D. Wu, L.Y. Chen, W. Lee, et al., Coord. Chem. Rev. 354 (2018) 74-97. DOI:10.1016/j.ccr.2017.06.011 |
| [23] |
X. Han, F. Hu, S.H. Liu, et al., Sens. Actuators B-Chem. 277 (2018) 55-61. DOI:10.1016/j.snb.2018.08.109 |
| [24] |
B. Ciubini, S. Visentin, L. Serpe, et al., Dyes Pigm. 160 (2019) 806-813. DOI:10.1016/j.dyepig.2018.09.009 |
| [25] |
L. Jiao, F.S. Long, J.N. Cui, et al., Chem. Commun. 54 (2018) 9198-9201. DOI:10.1039/C8CC04582H |
| [26] |
J. Atchison, S. Kamila, H. Nesbitt, et al., Chem. Commun. 53 (2017) 2009-2012. DOI:10.1039/C6CC09624G |
| [27] |
C. Shirata, J. Kaneko, Y. Inagaki, et al., Sci. Rep 7 (2017) 13958. DOI:10.1038/s41598-017-14401-0 |
| [28] |
J. Chen, X. Tan, S.L. Luo, et al., J. Innov. Opt. Health Sci. 11 (2018) 1850016. DOI:10.1142/S1793545818500165 |
| [29] |
S.L. Luo, X. Tan, S.T. Fang, et al., Adv. Funct. Mater. 26 (2016) 2826-2835. DOI:10.1002/adfm.201600159 |
| [30] |
X.Q. Meng, J.L. Zhang, Z.H. Sun, et al., Theranostics 8 (2018) 6025-6034. DOI:10.7150/thno.26607 |
| [31] |
X.Q. Meng, Y.T. Yang, L.H. Zhou, et al., Theranostics 7 (2017) 1781-1794. DOI:10.7150/thno.18437 |
| [32] |
A. Turksoy, D. Yildiz, E.U. Akkaya, Coord. Chem. Rev. 379 (2019) 47-64. DOI:10.1016/j.ccr.2017.09.029 |
| [33] |
C.J. Reinhardt, E.Y. Zhou, M.D. Jorgensen, et al., J. Am. Chem. Soc. 140 (2018) 1011-1018. DOI:10.1021/jacs.7b10783 |
| [34] |
J.A. Peterson, C. Wijesooriya, E.J. Gehrmann, et al., J. Am. Chem. Soc. 140 (2018) 7343-7346. DOI:10.1021/jacs.8b04040 |
| [35] |
B.S. Kim, B.L. Sui, X.L. Yue, et al., Eur. J. Org. Chem. 2017 (2017) 25-28. DOI:10.1002/ejoc.201601054 |
| [36] |
M.L. Li, R.S. Tian, J.L. Fan, et al., Dyes Pigm. 147 (2017) 99-105. DOI:10.1016/j.dyepig.2017.07.048 |
| [37] |
F.F. Xue, P. Wei, X.T. Ge, et al., Dyes Pigm. 156 (2018) 285-290. DOI:10.1016/j.dyepig.2018.04.008 |
| [38] |
H.S. Jung, J.Y. Han, H. Shi, et al., J. Am. Chem. Soc. 139 (2017) 7595-7602. DOI:10.1021/jacs.7b02396 |
| [39] |
C.S. Kue, A. Kamkaew, H.B. Lee, et al., Mol. Pharm. 12 (2015) 212-222. DOI:10.1021/mp5005564 |
| [40] |
M. Li, S. Long, Y. Kang, et al., J. Am. Chem. Soc. 140 (2018) 15820-15826. DOI:10.1021/jacs.8b09117 |
| [41] |
R.L. Watley, S.G. Awuah, M. Bio, et al., Chem.-Asian J. 10 (2015) 1335-1343. DOI:10.1002/asia.201500140 |
| [42] |
N. Shivran, M. Tyagi, S. Mula, et al., Eur. J. Med. Chem. 122 (2016) 352-365. DOI:10.1016/j.ejmech.2016.06.050 |
| [43] |
R. Ridhi, G.S.S. Saini, S.K. Tripathi, Sens. Actuators B-Chem. 255 (2018) 1849-1868. DOI:10.1016/j.snb.2017.08.213 |
| [44] |
G. Avsar, F.A. Sari, A.G. Yuzer, et al., Int. J. Pharm. 505 (2016) 369-375. DOI:10.1016/j.ijpharm.2016.04.023 |
| [45] |
F.Y. Lambrecht, K. Ocakoglu, O. Er, et al., J. Label Compd. Radiopharm. 59 (2016) 221-227. DOI:10.1002/jlcr.3395 |
| [46] |
Z.X. Zhao, P.S. Chan, H.G. Li, et al., Inorg. Chem. 51 (2012) 812-821. DOI:10.1021/ic201178e |
| [47] |
K. Li, L. Qiu, Q.Z. Liu, et al., J. Photochem. Photobiol. B 174 (2017) 243-250. DOI:10.1016/j.jphotobiol.2017.08.003 |
| [48] |
A.D. Gülmez, M. Göksel, M. Durmus, et al., J. Porphyrins Phthalocyanines 21 (2017) 1-8. DOI:10.1142/S1088424616501273 |
| [49] |
B.Y. Zheng, X.Q. Yang, Y. Zhao, et al., Eur. J. Med. Chem. 155 (2018) 24-33. DOI:10.1016/j.ejmech.2018.05.039 |
| [50] |
J.J. Wu, Z. Ye, F. Meng, et al., Talanta 181 (2018) 239-247. DOI:10.1016/j.talanta.2018.01.028 |
| [51] |
D.Y. Lee, K.M.K. Swamy, J.H. Hong, et al., Sens. Actuators B-Chem. 266 (2018) 416-421. DOI:10.1016/j.snb.2018.03.133 |
| [52] |
S.L. Shen, X.F. Zhang, Y.Q. Ge, et al., Sens. Actuators B-Chem. 256 (2018) 261-267. DOI:10.1016/j.snb.2017.10.103 |
| [53] |
W. Piao, K.J. Hanaoka, T. Fujisawa, etal., J.Am.Chem.Soc. 139 (2017) 13713-13719. DOI:10.1021/jacs.7b05019 |
| [54] |
M. Chiba, Y. Ichikawa, M. Kamayi, et al., Angew. Chem. Int. Ed. 56 (2017) 10418-10422. DOI:10.1002/anie.201704793 |
| [55] |
Y.S. Chou, C.C. Chang, T.C. Chang, et al., Biomed Res. Int 2013 (2012) 930281. |
| [56] |
J. Mei, Y.H. Huang, H. Tian, ACS Appl. Mater. Interfaces 10 (2018) 12217-12261. DOI:10.1021/acsami.7b14343 |
| [57] |
X.G. Gu, X.Y. Zhan, H.L. Ma, et al., Adv. Mater 30 (2018) 1801065. DOI:10.1002/adma.201801065 |
| [58] |
D.D. La, S.V. Bhosale, L.A. Jones, et al., ACS Appl. Mater. Interfaces 10 (2018) 12189-12216. DOI:10.1021/acsami.7b12320 |
| [59] |
C.J. Zhang, Q.L. Hu, G.X. Feng, et al., Chem. Sci. 6 (2015) 4580-4586. DOI:10.1039/C5SC00826C |
| [60] |
Y.Y. Yuan, C.J. Zhang, S.D. Xu, et al., Chem. Sci. 7 (2016) 1862-1866. DOI:10.1039/C5SC03583J |
| [61] |
D. Wang, H.F. Su, R.T.K. Kwok, et al., Chem. Sci. 9 (2018) 3685-3693. DOI:10.1039/C7SC04963C |
| [62] |
J.L. Zou, H.G. Lu, X.W. Zhao, et al., Dyes Pigm. 151 (2018) 45-53. DOI:10.1016/j.dyepig.2017.12.044 |
| [63] |
Y. Li, K.N. Wang, B. Liu, et al., Sens. Actuators B-Chem. 255 (2018) 193-202. DOI:10.1016/j.snb.2017.08.041 |
| [64] |
Y.L. Lv, M. Liu, Y. Zhang, et al., ACS Nano 12 (2018) 1350-1358. DOI:10.1021/acsnano.7b07716 |
| [65] |
J. Qi, C.W. Sun, H.Q. Zhang, et al., ACS Nano 12 (2018) 7936-7945. DOI:10.1021/acsnano.8b02452 |
| [66] |
H.D. Li, X. Zhou, J.L. Fan, et al., Sens. Actuators B-Chem. 254 (2018) 709-718. DOI:10.1016/j.snb.2017.07.082 |
| [67] |
W.B. Hu, M. Xie, H. Zhao, et al., Chem. Sci. 9 (2018) 999-1005. DOI:10.1039/C7SC04044J |
| [68] |
Z. Zheng, T.F. Zhang, H.X. Liu, et al., ACS Nano 12 (2018) 8145-8159. DOI:10.1021/acsnano.8b03138 |
| [69] |
Y. Liu, X.X. Hu, L.L. Wang, et al., Dyes Pigm. 145 (2017) 168-173. DOI:10.1016/j.dyepig.2017.06.003 |
| [70] |
M. Li, J. Xia, R.S. Tian, et al., J. Am. Chem. Soc. 140 (2018) 14851-14859. DOI:10.1021/jacs.8b08658 |
| [71] |
X.Y. Yang, D.Y. Wang, Y.H. Shi, et al., ACS Appl. Mater. Interfaces 10 (2018) 12431-12440. DOI:10.1021/acsami.8b00276 |
| [72] |
Z. Lv, L. Zou, H.J. Wei, et al., ACS Appl. Mater. Interfaces 10 (2018) 19523-19533. DOI:10.1021/acsami.8b05944 |
| [73] |
W.J. Hou, Y. Liu, Y. Jiang, et al., Nanoscale 10 (2018) 10986-10990. DOI:10.1039/C8NR01096J |
| [74] |
L.L. Rui, H.L. Cao, Y.D. Xue, et al., Chin. Chem. Lett. 27 (2016) 1412-1420. DOI:10.1016/j.cclet.2016.07.011 |
| [75] |
Y. Zhu, W.H. Lin, W. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1875-1877. DOI:10.1016/j.cclet.2017.06.017 |
| [76] |
J. Mou, T.Q. Lin, F.Q. Huang, et al., Biomaterials 84 (2016) 13-24. DOI:10.1016/j.biomaterials.2016.01.009 |
| [77] |
Z.Q. Xu, J.H. Chen, L.L. Hu, et al., Chin. Chem. Lett. 28 (2017) 1935-1942. DOI:10.1016/j.cclet.2017.07.018 |
| [78] |
C.B. Huang, L. Xu, J.L. Zhu, et al., J. Am. Chem. Soc. 139 (2017) 9459-9462. DOI:10.1021/jacs.7b04659 |
| [79] |
J.C. Xu, H.Q. Yuan, L.T. Zeng, et al., Chin. Chem. Lett. 29 (2018) 1456-1464. DOI:10.1016/j.cclet.2018.08.012 |
| [80] |
H.J. Lee, Y.G. Lee, J. Kang, et al., Chem. Sci. 10 (2019) 1000-1007. DOI:10.1039/C8SC04943B |
| [81] |
J.L. Zhu, P.P. Jia, N. Li, et al., Chin. Chem. Lett. 29 (2018) 1445-1450. DOI:10.1016/j.cclet.2018.09.002 |
| [82] |
D.F. Yue, M.L. Wang, F. Deng, et al., Chin. Chem. Lett. 29 (2018) 648-656. DOI:10.1016/j.cclet.2018.01.046 |
| [83] |
W.J. Qin, C.C. Xu, Y.F. Zhao, et al., Chin. Chem. Lett. 29 (2018) 1451-1455. DOI:10.1016/j.cclet.2018.04.007 |
| [84] |
H.L. Un, S. Wu, C.B. Huang, et al., Chem. Commun. 51 (2015) 3143-3146. DOI:10.1039/C4CC09488C |
 2019, Vol. 30
2019, Vol. 30